Initial Publication Date: August 10, 2007
Different Kinds of Reactions
Examples of phase diagrams and reactions in jpg, pdf and animated-pdf format are available.
A 5 page summary (Acrobat (PDF) 158kB Aug1 07) of this information is available, which can be used as a class handout.
Reactions among solid and fluid phases can be categorized in several different ways, based on what the reaction does, how the reaction progresses, or based on the nature of phases involved.
Categorization based on reaction effect
- Net-transfer reactions involve chemical components being "transferred" from one phase or set of phases to others (new phases are produced as old ones disappear). An example is:
anorthite = grossular + kyanite + quartz
Net-transfer reactions may be terminal reactions or tie-line flip reactions (discussed below). - Exchange reactions involve chemical components being exchanged between phases, so compositions change, but modes remain the same (no phases disappear and no new phases are produced). An example is:
Fe (in garnet) + Mg (in biotite) = Mg (in garnet) + Fe (in biotite)
Categorization based on reaction progress
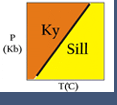
- Discontinuous reactions are those that occur at a particular temperature (for a particular pressure). For these, a curve (or line) can be drawn on a pressure-temperature (P-T) diagram. (See the kyanite=sillimanite reaction on the phase diagram shown below.) On either side of the curve, a different set of phases is stable. In a discontinuous reaction, products and reactants can only co-exist stably precisely at the equilibrium reaction conditions (on the reaction line in P-T space). Discontinuous reactions are always net-transfer reactions.
- Continuous reactions involve phases that may vary in composition. Such reactions are more common than discontinuous reactions because compositional variation, of either fluid or solid phases, is typical for geological materials. Continuous reactions occur over a range of conditions, so the products and reactants coexist stably over a range of conditions (but the compositions of the phases changes systematically as conditions change). Continuous reactions may be net-transfer reactions or exchange reactions.
Categorization based on phases involved
- Solid-solid net transfer reactions (often just called solid-solid reactions) occur among solid phases of differing composition. These phases may include elements found in fluids (H, C), but those elements are conserved in the solid phases so that no fluid phases (H2O, CO2) are involved as reactants or products. Solid-solid net transfer reactions can be continuous or discontinuous, and they may be terminal, or tie-line flip reactions (discussed below).
- Polymorphic reactions are a special type of solid-solid reaction that involves phases of identical composition. Classic examples are the reactions among the aluminum silicates (kyanite-sillimanite-andalusite; see the photomicrograph and phase diagram shown at right), the conversion of graphite to diamond at high pressure, and calcium carbonate (calcite-aragonite) equilibria.
- P-T diagram showing the (dehydration) reaction of brucite to periclase + H2O. Figure from D. Perkins.Devolatilization reactions are net-transfer reactions that involve the liberation of a volatile phase (H2O for dehydration reactions or CO2 for decarbonation reactions). Examples of a dehydration and a decarbonation reaction are:
muscovite + quartz = K-feldspar + sillimanite + H2O, (dehydration)
calcite + quartz = wollastonite + CO2 (decarbonation)
Because the entropy of a fluid is generally greater than the entropy of solid phases, fluids appear on the high-temperature side of most such reactions. If the fluid composition is fixed (stays constant), then devolatilization reactions are discontinuous, but if the fluid composition can vary as a result of the liberation of H2O or CO2, then the reactions will be continuous.
Curvature: The curves for dehydration or decarbonation reactions on a P-T diagram will have shallow slopes at low pressure because the volume of a fluid phase is much larger than that of solid phases. However, the compressibility of a fluid leads to a rapid decrease in volume as pressure rises, so the slopes steepen with rising pressure, leading to substantial curvature. Some (rare) reactions curve around and gain a negative slope at high pressure.
Sensitivity to fluid composition: The P-T position of a decarbonation or dehydration reaction changes if fluid composition changes. For a dehydration reaction such as:
muscovite + quartz = K-feldspar + sillimanite + H2O,
if the rock contains an H2O-rich fluid, the right-hand side of the reaction will be stable over a smaller range of conditions than if the rock contains an H2O-poor fluid (either because the fluid is diluted with CO2, or the rock is ''dried out'' and not fluid saturated).
Mixed-volatile reactions involve the liberation and/or consumption of more than one volatile phase, typically H2O and CO2. An example is:
tremolite + calcite + quartz = diopside + H2O + CO2
Mixed volatile reactions have the same general shape as dehydration and decarbonation reactions on P-T diagrams. (However, they are generally plotted on T-X diagrams instead of P-T diagrams. Click here for more discussion of different types of phase diagrams.) - Terminal reactions involve the creation of a new phase from two or three other phases, or (in the other direction) the decomposition of one phase into two or three others. They may be solid-solid reactions or they may involve a fluid phase. An example of a terminal reaction is:
chloritoid = staurolite + garnet + chlorite
At lower temperature (583°), chloritoid is stable for compositions that fall within the red, green and yellow 3-phase fields.
At higher temperature (584°), staurolite, garnet and chlorite are stable together and chloritoid is gone. So, the reaction takes place between 583° and 584° at 1 GPa.
AFM diagram projected from quartz, muscovite. Click for animated GIF showing loss of chloritoid. QuickTime version ( 67kB Apr2 07)
- Tie line flip reactions involve two phases becoming stable together that were previously unstable together, and vice versa. As with terminal reactions, they may be solid-solid reactions or may involve a fluid phase. An example of a tie-line flip reaction is:
chlorite + garnet = biotite + staurolite
AFM diagram projected from quartz, muscovite. Click for animated GIF showing tie-line flip. QuickTime version (Quicktime Video 261kB Aug8 07)
- Ion-exchange reactions (generally just called "exchange reactions") involve two phases that both share a solid solution exchange such as Na↔K or Fe+2↔Mg such as garnet, (Fe,Mg)3Al2(SiO4)3 and biotite, K(Fe,Mg)3(Si3Al)O10(OH)2 (both formulas are simplified). Reactions of this type are all continuous, and differ from the others kinds of reactions discussed above in that there is no real product or a reactant. Instead, across the complete range of conditions over which the two minerals are stable, both are changing in composition. Curves for these reactions are straight on a P-T diagram (but can only be plotted for specific compositions) and very steep (because there is essentially no change in volume associated with the reaction.
For Fe+2 and Mg exchange between garnet and biotite, the reaction can be written:
almandine-in-garnet + phlogopite-in-biotite ↔ pyrope-in-garnet + annite-in-biotite, or:
Fe3Al2(SiO4)3 + KMg3(Si3Al)O10(OH)2↔Mg3Al2(SiO4)3 + KFe3(Si3Al)O10(OH)2.
Identifying Possible Chemical Reactions in a Chemical System
Dmitry Dolivo-Dobrovolsky has developed Reactions, a small handy freeware for generation of all possible reactions between specified substances (pure minerals and components of solid solutions, oxides and chemical elements) with selection of the arbitrary set of linearly independent reactions. This program may be useful for education, for analysis of diagrams (petrogenetic grids and results of multi-equilibrium geothermobarometry), for balancing of reactions involving minerals with real compositions, for evaluation of solid solution end-members, etc.
Teaching Aids
- Metamorphic Mineral Assemblages ( This site may be offline. )
- Metamorphic Reactions, Isograds, and Reaction Mechanisms ( This site may be offline. )
- QuickTime version ( 67kB Apr2 07) of Cld-out terminal reaction animation - Dave Hirsch, Western Washington University
- QuickTime version (Quicktime Video 261kB Aug8 07) of Grt+Chl=St+Bt tie line flip reaction animation - Dave Hirsch, Western Washington University
- QuickTime version ( 70kB Apr2 07) of continuous dehydration reaction animation - Dave Hirsch, Western Washington University
- QuickTime version ( 103kB Apr2 07) of continuous solid-solid net transfer reaction animation - Dave Hirsch, Western Washington University
Problem Sets / Lab Activities
- Lab Activity: Calculating a Simple Phase Diagram: Diamond=Graphite - Dexter Perkins, University of North Dakota
- Introduction to Mineral Equilibria - Dexter Perkins, University of North Dakota
- Mass Balance and Mineral Reactions - Dexter Perkins, University of North Dakota
- Thermodynamic Calculation of Mineral Reactions I Lab (Microsoft Word 54kB Mar29 07) - This one week Excel-based exercise, provided by Dave Pattison at the University of Calgary, includes problems sets including the calculation of thermodynamic equilibria involving pure phases.
PDF version (Acrobat (PDF) 1.1MB Mar29 07)
Excel Key (Excel 51kB Mar29 07) - Thermodynamic Calculation of Mineral Reactions II Lab (Microsoft Word 48kB Mar29 07) - This Excel-based one week exercise, provided by Dave Pattison at the University of Calgary, includes problems sets involving equilibrium constants, activities and calculation of thermodynamic equilibria involving impure phases, and 'conventional' thermobarometry using the GTB program.
- Metapelites Lab (Acrobat (PDF) 160kB Mar29 07) - This one week exercise, provided by Dave Pattison at the University of Calgary, includes problems sets involving petrogenetic grids, AFM diagrams, bulk compositions, mineral assemblages and isograds, as well as the use of program Gibbs.