Needs and Opportunities
Nanoparticles in the Earth System: Missing Links?
"Earth is a complex system of interacting physical, chemical and biological processes, and provides a natural laboratory whose experiments have been running since the beginning of time" (Earth system science module from the Starting Point project). Earth system science considers Earth as a closed system (the "Blue Marble" floating in space) and focuses on the interactions among the "spheres" of Earth (atmosphere, hydrosphere, biosphere, lithosphere....). The focus is on energy and mass transport, storage, consumption and liberation among the components of the Earth system, and this transfer of energy and mass account for the chemical, physical, and biological work that is done by the planet. Much of this work is done through global cycling, e.g., the water cycle, carbon cycle, plate tectonics. The Earth system is heterogeneous, dynamic, complex and often chaotic. This requires observation of earth on all spatial scales from atomic to planetary, and temporal scales that may be instantaneous or span the breadth of geologic time ("deep time", 4.6 billion years). Temporal concepts are extremely important to Earth System Science, and it's important for models of Earth processes to include consideration of rates, fluxes, duration of events, and recurrence intervals. There is an increasing awareness of the co-evolution of the Earth system in terms of its physical and chemical properties and the evolution of life. And, impacts of humanity on the Earth system, and the realization that humanity is a geologic agent with potential to impart irreversible consequences on the operation of Planet Earth, has led to the concept of the Anthropocene.
Here's a metaphor: consider nanoparticles in the Earth system to be like cold dark matter in the universe: we know it must be there, but we don't know the whole story of how the Earth/Universe works unless we purposefully look for this matter to characterize and integrate into models and theories to holistically explain the nature of the world/universe around us. Similarly, the contributions of nanoparticles to the energetics of Earth systems (free energies, surface energies, reactivities, rates of reactions) can be compared to dark energy in the universe: although not readily evident, these energies contribute significantly to the driving mechanisms, processes and work of Planet Earth.
What's missing? Incorporation of nanoscience in Earth science research and teaching. Here are some key areas that could and should be addressed:
- Characterization of nanoparticles in the same manner minerals, rocks, soils, waters are analyzed on the macroscale.What's missing? Systematic and routine searching for nanoparticles in all parts of the Earth system to characterize their chemical and physical properties and interactions with biota and humanity. We now have a vast arsenal of Geochemical Analytical Instruments and Techniques (TEM, SEM, EDS, XRD, ToF-SIMS, SP-LA-ICPMS, AFM and many more) that are routinely used to observe and measure the composition, structure, morphology (size and shape distribution), chemical state, crystallography and crystallographic orientation. Consider the contribution of H.C. Sorby (1826-1908) the "Father of Petrography" and fluid inclusion analysis (and slaty cleavage studies) who stated: "In those early days people laughed at me. They quoted Saussure who had said that it was not a proper thing to examine mountains with [atomic force] microscopes, and ridiculed my action in every way. Most luckily I took no notice of them." Our ability to detect, collect, characterize and model nanoparticles today is every bit as revolutionary to our science as the application of the petrographic microscope was a century and a half ago. We have every opportunity to explore planet Earth on the nanoscale--and to ignore or avoid the nano-world is to accept that our models and theories of Earth will be incomplete and incorrect.
- Studies of phase equilibria. We are very familiar with the formulation: G = H - TS + PV. What's missing? The inclusion of surface energies into our understanding of the energetics of reactions that take place in nature or the laboratory. At the nanoscale, surface energies become large with respect to "bulk" energies and quantum effects take over! Also, Gibbs' Phase Rule (P + F = C + 2) is routinely taught in Mineralogy and Petrology classes, but at the nanoscale there is an open question about what is the definition of "phase" when dealing with thin (atomic layer scale ) coatings, or nanoparticles that may have only short range order.
- Studies of the surfaces of materials (a few atomic layers, nanometer-scale) compared to "bulk materials". What's missing? A consideration of surface properties of materials: composition, chemical state, atomic structure. The surface of materials is where all the action is when interacting with an external environment (liquids, gases, melts, biota...). Material surfaces may facilitate chemical reactions as catalysts, or they may inhibit reactions by forming an armoring layer. Materials dissolve or precipitate at material-fluid interfaces, and thus control the local geochemical environment. Free ions may sorb onto material surfaces, and this creates problems and opportunities with regard to environmental and health hazards. Cells may attach to materials, further impacting biogeochemical processes that are surface-mediated. It is rarely the case that the surface composition (a few atomic layers) is the same as the underlying bulk composition. There are many modern spectroscopies (Time-of Flight SIMS, Auger Electron Spectroscopy, X-ray Photoelectron spectroscopy, and related TEM, SEM, and AFM methods that can be more broadly applied to studies of the Earth system.
- Surface-mediated reactions: What's missing? A systematic exploration on the nanoscale of the role of surface-mediated reactions such as catalysis, sorption, solution/precipitation, buffering capacity, REDOX, and interfaces with biota (e.g., biomineralization, influence of microbial "exopolymeric substance", EPS).
- Sampling and lab practices: What's missing? Detection of nanoparticles may be confounded for numerous reasons, and may be missed when developing models of chemical reactions and pathways. 1) Some nanoparticles may be ephemeral, although important phases, in chemical reactions. An example is the occurrence of "green rust" that only occurs in nature under very reducing conditions. Any exposure to ambient atmosphere will result in nearly instantaneous oxidation of the green rust. So, if care is not taken in sampling procedures to isolate the sample this phase will be reacted out and not available for subsequent analysis in the laboratory. 2) Nanominerals are minerals that only occur on the nanoscale (100nM<). It is very possible that these phases may be an important part of a geochemical system, but may be completely overlooked if only micro- to macro-scopic observations are made. The search for nanomaterials in the Earth system has to be purposeful and systematic. You don't know what you're missing unless you take the time to look. Yogi Berra, that great 20th Century philospher, had it right: "You can see a lot just by looking!"
- Earth system science is increasingly utilizing the tenets of Complex System Behavior to model Earth processes. This includes consideration of feedback loops, strongly interdependent variables, chaotic behavior, multiple (meta)stable states, self-organized criticality, emergent phenomena, fractal geometry, and sensitivity to initial conditions. What's missing? A consideration of the role of nanoparticles as they define initial conditions of a system, multiple pathways of reactions, emergent phenomena that will be different on the nanoscale compared to the macroscale, fractal dimensions that may vary significantly (as rods or sheets) compared to bulk (3D, volume) materials.
- Mass budgets and biogeochemical cycling:What's missing? Inclusion of estimates or measurements of nanoparticles in models of mass transport along the pathways and among reservoirs in the Earth system. See: Hochella, M., Aruguete, D., Kim, B., and Elwood Madden, A., 2012, Naturally occurring inorganic nanoparticles: general assessment and a global budget for one of earth's last unexplored major geochemical components, Pan Stanford Publishing Pte. Ltd., Nature's Nanostructures, 1-42 p. as an example.
- The "Big Questions" to be addressed by future research in the Earth Sciences: The National Research Council (2012) outlined "New Research Opportunities in the Earth Sciences at the National Science Foundation", National Academy Press. What's missing? A systematic recognition that nanomaterials impact virtually all processes at work in the Earth system. Here's a big question: What is the origin of life? There is a solid body of evidence that suggests that mineral surfaces have served as the template for sorbing, concentrating, and synthesizing complex organic macromolecules. Nanoparticles that emanate from submarine "black smokers" produce chemical nutrients that provide energy sources for microbial communities. Early life could have been sustained by nanoparticles in this environment.
- The role of nanoparticles in Earth history: What's missing? The realization that nanoparticles have always been present in the Earth system, and probably played a major role in significant episodes in Earth history. An example is the "Great Oxygenation Event" in the Precambrian. The GOE certainly occurred in response to photosynthesis by cyanobacteria that produced free oxygen. This oxygen then reacted with Fe+2 in the reduced ocean to form mineral nanoparticles of iron oxides (hematite, magnetite) that precipitated out of seawater to form the global occurrences of banded iron formations. See: Rasmussen, B., Krapež, B., Muhling, J. R., and Suvorova, A., 2015, Precipitation of iron silicate nanoparticles in early Precambrian oceans marks Earth's first iron age: Geology, v. 43, no. 4, p. 303-306.
- The increased incidence of engineered and incidental (anthropogenic) nanoparticles in the environment: What's missing? A consideration of the ethics of purposefully releasing nanoparticles to the environment in an attempt to "control" Nature. Who makes the decision? Who are the stakeholders? Who has the power? Who can get hurt? Are the actions reversible? Are they defensible--to scientific peers, to the legal system, to the public? What are the consequences? Recall chaos theory and sensitive dependence on initial conditions. What can be done to prevent a "runaway" situation if we use nanoscience to alter natural processes and rates?
-
The future of the Earth Sciences and Earth (and Environmental) Scientists: What's missing? YOU and YOUR STUDENTS. The nanoscience/technology revolution has been in progress since the early 1960's (see below). The Earth and Environmental Sciences have not participated in this revolution to the extent of our colleagues in our sister STEM disciplines. Exciting new frontiers of Earth and Environmental Science are open for exploration at the nanoscale. There are great needs for a workforce trained to engage research and applications of nanoscience/technology. This is an appeal to faculty to take the initiative to learn a bit more about emerging research in nanoscience, integrate this exciting new science into your coursework, and help students recognize potential career pathways in nano-related jobs.
Opportunity, Need (and Urgency!) in the Earth and Environmental Sciences
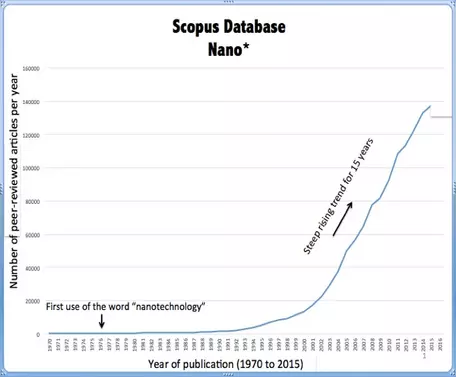
The Earth and Environmental Sciences are far behind other STEM disciplines in engaging the nanoscience revolution. It is true that many sub-disciplines in the geosciences have long made contributions that could be considered nanoscience in areas such as clay mineralogy, colloid geochemistry, biomineralization and many more. A literature search (1970-2015, figure on the left) by Michael Hochella has demonstrated that there has been steady growth in publications to a recent rate of about 200-300 publications/year. However, over the same time period, publications that relate to Nanotechnology have experienced a very steep rise to recent publication rates approaching ~140,000 articles/year! The Earth and Environmental Sciences are far behind the curve in addressing this emerging field of research. There are great opportunities for geoscientists from many sub-disciplines (Mineralogy, Petrology, Geochemistry, Hydrology....) to make fundamentally new contributions to:
- Characterization of natural, engineered and incidental nanoparaticles as they occur throughout the Earth system; because there has not been a concentrated effort to look for, identify, and characterize chemical and physical properties of nanoparticles, we really know very little about the diversity of these particles in the Earth system;
- The role of nanoparticles of all types in the Earth system, and their contributions to mass and energy transport and storage in global biogeochemical cycling;
- Demonstrations of how the physical and chemical properties of materials change from the nano- to the meso-scale;
- Impacts of nanoparticles on biosystems (ecosystem to microbial impacts) and on human health; and,
- Demonstrating how these size-dependent properties expressed on the nanoscale can be harnessed to help develop next-generation materials in service to society.
The geosciences tradtitionally study the open, dynamic, heterogeneous and complex components of the Earth system, and routinely integrate data across the physical, chemical, biological sciences and engineering disciplines. These attributes place the geosciences in a great position to contribute to the growing field of nanoscience/technology. (This is closely aligned with NSF's "10 Big Ideas" and the need to engage Convergent Science
The challenge for the geosciences is that nanoscience is not widely taught in the geoscience curriculum. Nanoscience is largely not covered in traditional Mineralogy, Petrology and Geochemistry textbooks. Few faculty have training in the methods and applications of nanoscience, so consequently nanoscience is rarely covered in formal coursework. We are not in a position to train the large number of geoscientists who will be needed to address questions related to the occurrence of nanoparticles in the environment. There is a real need (and urgency) for the Earth and Environmental Sciences to:
- Train faculty and keep them current in the methods and applications of nanoscience to the Earth system;
- Develop curricular materials and instructional resources that can be embedded into Earth and Environmental Science courses across the curriculum; and
- Undertake systemic training programs at both the undergraduate and graduate level to prepare today's students for next-generation professional careers in nanoscience.
We are missing a huge opportunity to serve our students as they prepare for their careers, expand our curriculum to be and to engage some really interesting and important science.
Topics in Nanoscience of Importance to the Earth and Environmental Sciences
Hochella et al., (2008) have made the case for the importance of nanoscience in the Earth and Environmental Sciences. Formation, release, environmental transformation, transport, fate and impact of natural, incidental and anthropogenic nanoparticles impact all components of the Earth system. Nanoscience could and should be part of the research and education mission across the discipline/curriculum in the Earth Sciences. Earth science fields for which nanoscience and technology are particularly important include:
- Petrology (Igneous, Metamorphic, Sedimentary)
- Geomechanics
- Field Geology and Geophysics
- Atmospheric Science
- Geochemical Transport, Trace Element Mobility
- Biomineralization, Mineral-Microbe, Biogeochemistry
- Mineralogy, Crystallography
- Crystal Growth
- Environmental Geochemistry
- Mineral-Water Interface Geochemistry
- Weathering, Dissolution
- Soil Science, Clay Mineralogy
- Colloidal Science
- Global Biogeochemical Cycling
- Aqueous Geochemistry
- Geologic Resources (Ores, Fuels)
- Incidental Materials in the Environment
- Engineered Materials in the Environment
- Geoethics
Further Reading
Hochella, M. F., Lower, S. K., Maurice, P. A., Penn, R. L., Sahai, N., Sparks, D. L., and Twining, B. S., 2008, Nanominerals, mineral nanoparticles, and earth systems: Science, v. 319, no. 5870, p. 1631-1635.


![[reuse info]](/images/information_16.png)

![[creative commons]](/images/creativecommons_16.png)