The Story of Big Moose Lake: Resilience, Tipping Point, and Restoration
Bill Stigliani, Center for Energy & Environmental Education, University of Northern IowaSummary
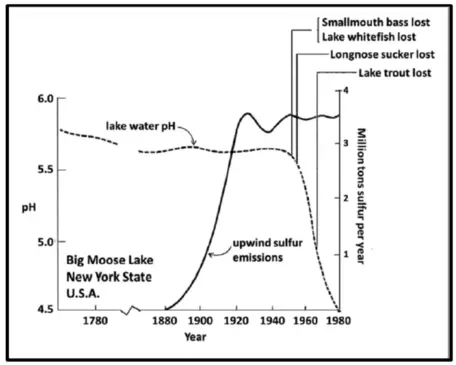
Big Moose Lake was one of the few lakes where accurate, simultaneous data existed for long-term trends in pH, upwind SO2 emissions, and fish populations. As illustrated in the figure, these data provide a concise glimpse of the etiology of events that led to the acidification of the mountain lakes. Notice that the pH of the lake water remained essentially constant (except for a slight decline due to natural senescence) over the period from 1760 to about 1950. Then within the space of 30 years, the pH declined more than 1 whole pH unit (corresponding to a factor of 10 increase in acidity). It can also be observed that the rapid onset of pH decline was not synchronous with the onset of acidic sulfur emissions from the industrial heartland. Rather, this "tipping point" in pH occurred 70 years after SO2 emissions began and 30 years after the emissions peaked around 1920. Why the delayed response?
A lake receives most of its water not from direct wet precipitation on its surface, but rather from runoff and groundwater flow through the watershed soils draining into the lake. The soils buffer a lake against acidification because they possess what soil chemists call "Cation Exchange Capacity" (CEC). It is the CEC that serves to neutralize acidic inputs that would otherwise flow unhindered into the lake. [See the accompanying class activity "Nature's Alka-Seltzer" for a more detailed discussion of lake water buffering by watershed soils, and why tipping points in pH occur.] A lake's resilience to acidification is determined by the size of the watershed's CEC. Even relatively well-buffered watersheds can lose their CEC over time when inputs of acid rain are high enough to surpass the rate at which the CEC can be naturally replenished. When the CEC declines to about 5% of its original capacity, the lake is highly vulnerable to the kind of rapid acidification experienced by Big Moose Lake.
The risk of lake acidification in the Adirondacks has been mitigated over recent decades as a result of two factors. One was the enactment of Title IV of the Clean Air Act Amendments of 1990, which specifically targeted acid rain by mandating upwind sources to significantly reduce their sulfur emissions. This has been achieved through a highly successful, market-oriented cap-and-trade system with active participation by the utilities industry. Over the period from 1980 to 2012, annual SO2 emissions from coal-fired power plants declined from 17.3 tons to about 3.3 tons (81% reduction).
The second factor has been the natural replenishment of CEC in the soil by addition of base cations from the weathering of bedrock. Recent studies suggest that with the continued decline of acid rain inputs and the increase in CEC, many lakes are gaining back their resilience and the ability to mitigate the impacts of acidification-stress to aquatic biota. The return of trophy-sized trout to some lakes and streams that had become fishless is one manifestation of this comeback.Individuals with expertise/responsibilities in the following areas have helped create the case study:
- Soil scientists, limnologists, and air pollution modelers
- The Environmental Protection Agency/Clean Air Act Amendments of 1990
- Utilities industry/participation in cap and trade transactions to reduce emissions
- New York Department of Environmental Conservation
- The Adirondack Council
Key teaching points:
- Limits to resilience. Ecosystems have resilience, but the resilience has limits. When limits are transgressed, a tipping point causing a major disruption in ecosystem function may occur.
- Resiliency and time-delayed impacts. The resilience of an ecosystem to an external stressor delays the time between the onset of the stressor and the occurrence of the tipping point. The greater the resilience, the longer the delay and vice versa. High resiliency can mask the risk posed by the stressor.
- Non-linear, surprising outcomes. Inputs of a stressor (e.g., a chemical pollutant) are typically linear over time, but the response of the ecosystem can be strongly non-linear. Non-linear behavior is manifested when a system reaches its tipping points. The occurrence of the tipping point can come as a complete surprise due to the delay in time between cause and effect.
- Early warning. In order to attain early warning about time-delayed impacts on ecosystems caused by inputs of external stressors, capacity controlling properties (CCPs) in the ecosystem that buffer against the stressors need to be identified and monitored.
- Restoration and policies. Restoration of ecosystems requires good policies that reduce or eliminate the stressor so that processes of natural replenishment are able to build up resilience.
How this example is used in the classroom:
By and large we are taught to think that the future will be merely a linear extrapolation of the past. In contrast, the example of Big Moose Lake highlights non-linear behavior and tipping points in the functioning of ecosystems. Students will better understand "systems thinking," which grasps the idea that an important quality of any interacting system is non-linear outcomes.
References
- W. Stigliani (1988). Changes in valued capacities of soils and sediments as indicators of nonlinear and time-delayed environmental effects. Environmental Monitoring and Assessment 10:245-307.
- W.M. Stigliani (2002). Contaminated lands and sediments: chemical time bombs? Pages 98-115 in T. Munn and I. Douglas (eds.), Encyclopedia of Global Environmental Change, Volume 3, Causes and Consequences of Global Environmental Change. Chichester, UK: John Wiley & Sons Ltd.
- C.T. Driscoll, K.M. Driscoll, K.M. Roy, M.J. Mitchell (2003). Chemical responses of lakes in the Adirondack Region of New York to declines in acidic deposition. Environmental Science & Technology 37(10): 2036-2042.
- National Acid Precipitation Assessment Program (2011). National Acid Precipitation Assessment Program Report to Congress 2011: An Integrated Assessment, 144 pp.
- Webpage of Professor Charles Driscoll at Syracuse University has update on current and projected status on acid-impaired lakes in the Adirondack Mountains. Go to: https://www.researchgate.net/profile/Charles_Driscoll/publications and click on link "Current Work on Acid Rain"
Supporting Files
Download this real-world example description: The Story of Big Moose Lake: Resilience, Tipping Points, and Restoration (Acrobat (PDF) 382kB Apr11 14)
![[creative commons]](/images/creativecommons_16.png)