For the Instructor
These student materials complement the Renewable Energy and Environmental Sustainability Instructor Materials. If you would like your students to have access to the student materials, we suggest you either point them at the Student Version which omits the framing pages with information designed for faculty (and this box). Or you can download these pages in several formats that you can include in your course website or local Learning Managment System. Learn more about using, modifying, and sharing InTeGrate teaching materials.Student Reading: Energy from Biomass
Learning Goals: Students will be able to:
- Recount the history of the use of biofuels during the course of human cultural development, including the impact on forests and whales.
- List and explain the impacts of biofuels on human health and air quality.
- Distinguish between alcohol fermentation and methane production from feedstocks, noting the different underlying biological processes and approaches to production.
- Evaluate the production of alcohol from sugar cane and corn, including the economics, energy efficiency, and ecological impact of each feedstock.
- Use data collected from experimentation to see how different feedstocks determine the biofuels produced under anaerobic conditions.
- Use published data to evaluate the efficacy of using land for biofuel production versus solar electric production.
Introduction
Human civilization took a big step forward when it learned how to use fire. Widespread use of fire dates to around 400,000 years ago. Hominids prior to modern humans may have used fire as early as 1.7 million years ago. Fire provided thermal energy to warm human bodies on chilly nights and to cook food. The cooking process killed pathogens and parasites in the food. It also made the food easier to digest. Fire also illuminated the night and may have scared off potential predators. The fuel for fire was dead plant material (we call any living or dead material biomass). Fire was the first environmental energy subsidy used by humans — the first time humans were able to benefit from energy other than what they got from their diet or from the sunshine on their cold backs.
Another important non-food human use of biomass was fiber for clothing and building protective structures. These uses made durable products, unlike firewood, which was rapidly consumed. When humans moved from hunting and gathering to agriculture, they changed the environment to accommodate that change. Slash-and-burn agriculture depended on burning large tracts of land to clear for planting crops. Ash, the residue from the burned trees and brush, enriched the soil with nutrients to support the crops. This style of agriculture is still practiced by indigenous people in tropical regions around the world. It also has taken on industrial proportions in places like Indonesia where fires are illegally set to destroy forest for the subsequent planting of coconut palms. The coconut palms produce the profitable coconut oil.
The energy in biomass comes from the sun. Sunlight is captured by green plants in the process of photosynthesis. Photosynthesis consists of a series of complex chemical reactions, but it can be thought of as having two main parts. The light reaction of photosynthesis is where sunlight is converted into chemical energy. It requires sunlight to excite electrons associated with a green pigment called chlorophyll. The energy in the excited electrons is used to create an intermediate concentration gradient of ions whose energy is then used to create a a chemical energy-transporting molecule called ATP (Adenosine Triphosphate). ATP is the main energy currency of life. The excited electrons have to come from somewhere, and that place is the simple molecule of water, H2O. To provide the electrons, water has to be split and this releases O2 as a waste product. The evolution of photosynthesis some 2.4 billion years ago literally changed Earth! It is the reason our atmosphere is now about 21% oxygen and can support higher life forms.
The first part of photosynthesis, the light reaction discussed above, is what provides the energy to do the second part, called the Calvin Cycle or dark reaction (because it can take place in the dark if energy is provided). The purpose of the dark reaction is to take carbon dioxide from the atmosphere and "fix" it into simple sugars. Those simple sugars can be used to store energy, or to power the production of other essential molecules such as cellulose, proteins, fats, oils, and thousands of other products.
The overall equation for photosynthesis is: energy (sunlight) + 6 CO2 + 6 H20 -> C6H1206 + 6 O2. The C6H1206 stands for glucose, a simple sugar. Humans rely entirely on photosynthesis for the oxygen we breathe and for the food we eat. It may be less obvious, but photosynthesis is also the basis for the fossil fuels that we have exploited since the industrial revolution. Coal, petroleum, natural gas, peat, shale oil, and tar sands (bitumen) are all fossils of biomass long since dead. These fossil fuels are all hydrocarbons, changed by time, pressure and heat from the original carbohydrates produced by photosynthesis millions of years ago. The problem is that we are using fossil fuels at a much greater rate than can be replaced by photosynthesis. This means we will eventually run out, and it means that we are translocating millions of tons of stored carbon from the crust of Earth to the atmosphere and the ocean. This is the basis for global climate change and the acidification of the seas.
Note that general formula for combustion of wood is the equation for photosynthesis in reverse: C6H1206 + 6 O2 -> 6 CO2 + 6 H20 + energy (heat). So what photosynthesis does is reversed by combustion.
Energy from biomass as a sustainable technology
Burning biomass for thermal energy is a hallmark of human civilization. But even before the Industrial Revolution, some civilizations overused biomass and caused severe environmental deterioration as early as 700,000 years ago. But that early forest destruction was mostly for agriculture.
Many people view production of energy from biomass as an important alternative to fossil fuels. The technologies range from the simple burning of wood to elaborate schemes to extract oil from algae. The argument for using biomass as fuel is that it is based upon photosynthesis and is therefore net-neutral to the carbon budget. That is, CO2 is removed from the atmosphere by photosynthesis and returned at about the same rate when the biomass is burned. In contrast, burning fossil fuels is not a balanced process and results in the accumulation of CO2 in the atmosphere and ocean.
Burning biofuels may be a very dirty business. Most biomass fuel is a dried solid. And most solids produce a residue of ash when they burn. Often that ash is distributed to the atmosphere in the hot gases of combustion. In addition, combustion is often incomplete, producing toxic and carcinogenic compounds. Catalytic converters may be fitted to the flue of biomass-burning stoves to help complete the combustion process. These are ceramic honeycombs coated with platinum or palladium. These metals provide a place for finishing the combustion process. This has the added benefit of producing more heat.
Burning wood, grasses, and manure for energy
All human cultures evolved with some source of biomass for combustion. In the United States and Europe it was mostly wood that played this role. In Asia, rice stalks and leaves are still important biomass fuels in many places. In much of India wood is scarce, and manure from cattle that graze on grass is important cooking and heating fuel (it is also used in construction as a mortar, and as fertilizer).
In Northern Europe, Ireland, and Scotland, peat is harvested by hand from wetlands and dried and burned in stoves for home use. There are also power plants that run on industrial-scale peat harvesting. Peat is composed of dead plant tissue that becomes part of the water-saturated soils of bogs and tundra. Since the soils remain waterlogged year-round, the plant material (detritus) lacks oxygen needed for rapid decomposition. The anaerobic decomposition process produces organic acids that, along with the tannins from the plants themselves, create a low pH (acid) environment that preserves the peat from further decomposition. Note that peat is a transitional fuel, being highly aged biomass. Some classify it as fossil fuel. The rate of renewal does not keep pace with removal, so in this way it does fit the fossil fuel description. It takes centuries for peat to form. In some areas peat accumulates at a rate of about 1 mm per year. If a 1-meter-deep block of peat is harvested, it will take 1,000 years for natural replacement, as there are 1,000 mm in a meter. In contrast, loblolly pine trees are grown in the southeastern part of the United States on a fifteen-year rotation between harvests.
Processed versions of those natural items listed above are gaining wide use. Those products provide a fuel of higher energy density and a size that is easier to handle and burn. They also take advantage of byproducts of industrial processes. For example, pellets made from sawdust and cornstalks are used in home stoves and industrial-sized power plants to produce electricity.
Wood pellet stove diagram from brighthub.com
All of these forms of biomass have the bulk of their energy stored in the molecule called cellulose. Cellulose is the most common form of organic carbon (carbon associated with molecules of life) in the biosphere. It is a twisted string of simple sugars that is very strong — it is the main component of wood. It is only slightly different from starch in structure, and like starch is rich in energy that is released in combustion with oxygen.
An enzyme called cellulase is required for the digestion of cellulose. It is only produced by microbes, and large animals with diets rich in cellulose (herbivores like cows and horses) have mutualistic associations with such microbes that live in their digestive systems. Despite the assistance of these microbes, dung produced by most herbivores consists of much partially digested cellulose, hence it is still rich in energy, enabling its use as fuel, discussed above.
Using biomass to produce alcohol for fuel
Another approach to using biomass for fuel involves modification to produce a liquid or gas from the solid parent material. The liquid fuels offer several advantages over their solid counterparts. Liquid fires can be started or stopped almost instantly. They contain more energy per unit mass, and are easy to transport. They tend to burn cleaner than solid fuels. They also can fuel vehicles like cars and trucks.
People of various cultures discovered the process of fermentation to make ethanol. Brewing alcoholic beverages was part of Ancient Greek and Chinese culture. So production of alcohol from biomass is certainly one of the oldest technologies known.
Ethyl and methyl alcohols are produced in the process of fermentation of biomass. Fermentation is a natural process that occurs in environments that lack free oxygen. Recall the equation for combustion given above: C6H1206 + 6 O2 -> 6 CO2 + 6 H20 + energy (heat). With only slight modification, it is also the summary equation for the process of cellular respiration in the presence of oxygen. This is how we and all other higher animals and plants get energy from biomolecules like sugar. The biological form of combustion (respiration) is: 36 ADP +C6H1206 + 6 O2-> 6 CO2 + 6 H20 + energy (heat) + 36 ATP (the real yield in practice is more like 30 ATP, but that is a long story).
Long before photosynthesis had enriched our atmosphere with oxygen, cells needed to get energy from biomolecules. And there are many places on Earth still lacking free oxygen: wet soils, lake and ocean sediments, etc. When free oxygen is not present, microbes rely upon anaerobic respiration. This releases much less energy than aerobic respiration because oxygen is really good at removing low-energy electrons produced by aerobic respiration. So the end products of anaerobic respiration still contain lots of energy to burn.
The summary formula for fermentation (as done by yeast) of sugar is: 2ADP + C6H1206 -> 2CO2 + 2 C2H5OH + energy (2 ATP). The ethyl alcohol produced in this reaction is a clean-burning fuel, but still a source of CO2 pollution.
In the United States most ethanol production for fuel (as opposed to drink) is done by fermenting corn. The fermentation process produces a product that is about 15% alcohol and 85% water. Fermentation stops at that point as the buildup of ethanol is poisonous to the yeast. To be used as a fuel, the alcohol has to be separated from the water by the process of distillation. Distillation involves using a low heat that will boil off the alcohol, but not vaporize much water. The boiling point for ethanol is 78.4o C. and for water is 100o C. So the temperature in the still must be kept between those two temperatures. The alcohol vapor is collected and cooled to make the liquid ethanol. Heating the still requires energy that is usually provided by fossil fuels. The alcohol distillation industry was well developed for the production of beverages long before gasohol came on the scene in the 1980s.
Calculations for the entire life cycle of corn-to-ethanol production reveal only modest energy gains. For every 1 unit of energy spent to produce corn ethanol, only 1.3 units are returned. Some suggest that it would be better to use the corn to feed people rather than internal combustion engines.
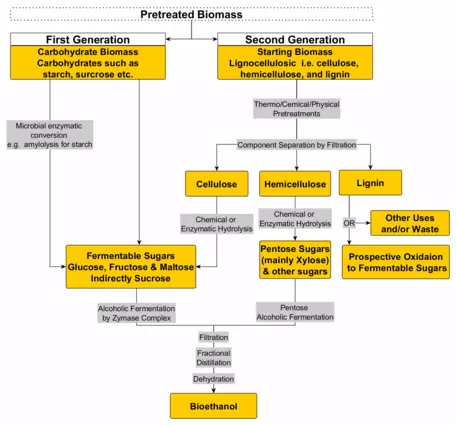
diagrams of corn ethanol production
Ethanol production from corn is big business. Estimates for 2013 indicate that 40% of the US corn production went to making ethanol, 45% went to animal feed, and 15% went to making food products for direct consumption by humans Article challenging the use of corn-ethanol. Note that corn is also the largest crop in the United States, followed by soybeans, wheat, and grass (lawns). The ethanol is used as a gasoline additive that provides a cleaner burn in automobile engines, as well as a source of more energy to run the engine.
Currently E10 blend (10% alcohol) is the main form of gasoline on the US market and has been in use since the early 1980s. Ethanol is added to gasoline for two reasons. First, it replaces Methyl Tertiary-Butyl Ether (MTBE) as an additive to give a cleaner burn. Without MTBE or ethanol, car engines produce smog-forming pollutants (organic and nitrogen compounds). In addition, the ethanol is a fuel, and thus replaces a portion of the gasoline that comes from petroleum.
In 2010 the US EPA authorized the use of E15 blend. The idea is that ethanol from biomass is more sustainable than gasoline from petroleum. But there are important objections to using ethanol for energy. The massive use of corn for fuel and animal feed has reduced the supply of corn for people to eat. And that has driven up the price of corn. This is having a devastating effect on poor people in developing nations such as Mexico that have come to rely upon imported corn.
The kind of corn agriculture practiced in the United States is not sustainable. Huge monocultures (single crop of the same variety) of corn require pesticides to kill insects, herbicides to kill weeds, water for irrigation, and vast quantities of nitrogen fertilizers. Those nitrogen fertilizers are made by a process that uses large quantities of fossil fuels. Much of the fertilizer applied to the corn washes away and into the Mississippi River. The river carries it to the Gulf of Mexico, where the fertilizer promotes the growth of algae. When the algae die, they sink to the bottom waters, and the decomposition processes uses up the available oxygen. This produces a "dead zone" where higher animals like fish cannot live. The herbicides and pesticides are pollutants that degrade soil, air, and water. Most of the corn is also genetically modified. This controversial process may also damage the environment.
Car and boat owners are also less than thrilled about ethanol in gasoline. The alcohol damages rubber hoses and engine seals. This is a larger problem in older vehicles not designed to withstand the ethanol. The problem is more acute for boat owners as the ethanol attracts water from the moist marine air. The water-gas-ethanol mix produces a gooey mass in fuel tanks and lines, requiring expensive repairs. The problem is worse when ethanol and MTBE are accidentally mixed. When MTBE and alcohol mix.
Cellulosic Ethanol
Instead of using corn, cellulose can be used to produce ethanol. The feedstock (biomass) used in this process is non-food crops. A variety of grasses, sawdust, biosolids from municipal wastewater plants and just about any cellulose-rich feedstock can be used. Switch grass is a native US prairie species with particular promise. Starch and sugar are easy for microbes to digest. Cellulose requires special enzymes to break the cellulose down into sugar before it can enter the fermentation process. Cows, deer, sheep and all other animals that eat high cellulose diets have digestive tracts evolved to support microbial communities that digest cellulose with the enzyme cellulase. The industrial production of cellulosic ethanol starts with a process that mimics the guts of herbivores.
Cellulosic ethanol boasts a much higher return on energy investment than that from corn. Estimates vary widely, but the range is for every unit of energy invested, 2–12 units of energy in the form of ethanol are returned. Despite the obvious advantages of cellulosic ethanol over corn alcohol, there is a lot of momentum to overcome to make the switch. First, it is costly to supply the enzymes, acid, and heat to run the process. Second, corn farmers have enjoyed many years of subsidies from Congress. There is also a large built infrastructure to support the industry. Corn ethanol plants can be converted to use a cellulose feedstock, but at considerable cost and re-engineering. Iowa is a center of corn production. It also hosts the first contest in US presidential primary elections. And the corn industry spends millions of dollars each year to lobby Congress. So the politics of changing to cellulose will be difficult.
Ethanol from sugar cane
Brazil produces its ethanol from sugar cane instead of corn. Sugar cane is a very productive plant. Much more of its biomass can be converted to ethanol than is the case for corn. For corn, it is only the starch in the kernels that can be used for effective fermentation. The sugar cane-to-ethanol industry in Brazil has much higher energy conversion efficiency than corn. For every unit of energy invested, 1.8 units of energy are returned in ethanol.
Ethanol has been mixed into gasoline in Brazil since the late 1920s. And in the 1980s, cars were introduced to that country that ran on 100% alcohol. More common today are vehicles that can use any mixture of gasoline and ethanol. These flex-fuel vehicles are also being marketed in the United States.
Articles about Ethanol Production in Brazil and US
Burning biogas for energy — landfills and methane generators
Methanogens are a functional class of microbes that uses a variety of biomolecules as a source of energy to make ATP. They flourish in the absence of oxygen, hence they are called anaerobic bacteria. Their waste product is methane (CH4). Methanogens include several different types of bacteria that specialize on the various different types of molecules encountered as living material decomposes. Generally these methanogens are a community of different bacteria that work together to break-down large complex biomolecules to smaller ones. Lipids (fats, oils, waxes), complex carbohydrates (cellulose and other plant fibers) and proteins are all such large molecules that feed the methanogens. Each step along the way, CH4is produced as an energy-rich waste product. CO2 is "reduced" to methane (CH4) as the processes proceed.
Methane is the primary constituent of the fossil fuel called "natural gas" so you know that like ethanol, it contains much usable energy. The environments that support methane production are anaerobic (no free oxygen is available). These environments also support the growth of microbes that use other pathways to use the energy of biomolecules. One set of bacteria produce H2S (hydrogen sulfide) as a byproduct. These anaerobic sulfur-reducing bacteria use the oxygen in SO4 to do the same job that free oxygen does for aerobic organisms. H2S is a deadly gas for humans. When it combines with free oxygen, it forms H2SO4, an air pollutant, and a powerful acid. Thus methane is typically contaminated with H2S, and it should be cleaned of this before being burned. Passing the gas mixture through a caustic solution (high pH) of NaOH (sodium hydroxide) is a way to clean up the methane before use.
Marco Polo wrote of covered sewage tanks in China; the practice of collecting methane there may date back several millennia. Assyrians used biogas to heat water during the 10th century BCE. An industrial scale digester was built in India in 1859. Exeter, England, fired its streetlights with biogas from a sewage plant built in 1895.
Biogas flourished in China during the 20th century, with 7 million small facilities in operation by 1999. In the 1970s biogas-fueled buses began operating in that country. Today some 60% of China's bus fleet runs on biogas. (http://www.apvgn.pt/fotos/fotos_pitorescas.html)
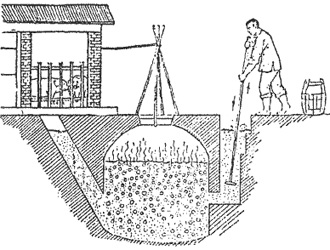
simple biogas digester design adaptable for use on farms
Municipal sewage plants in the United States experimented with biogas digesters in the 1930s. The Los Angeles Sanitation District runs a large biogas plant fueled by the biosolids produced in the wastewater treatment plant. Using municipal waste in anaerobic digesters has the added benefit of stabilizing the solids for use in soil enrichment. The term "stabilizing" means that residue of the anaerobic process will slowly decompose in the soil, and will not release offensive odors.
Livestock operations, dairy farms, breweries, and food processing plants are other industries that produce large quantities of biosolids that could be used for biogas production. The biogas they produce is used for heating and to make electricity by running generators.
Municipal landfills receive everything that people discard. This includes lots of biosolids in the form of paper, wood, food scraps, and yard waste (grass clippings). When a section of landfill has reached its design capacity, it is covered with a thick layer of soil. The anaerobic conditions of decomposition lead to production of methane. This may be a dangerous situation, as methane may seep through the ground into adjacent homes and buildings and cause fires and explosions. So wells are drilled into the landfills to release the methane. It is simple then to capture the escaping gas and put it to work.
Read about landfill gas facility in Edmonton, CA
Challenges for biogas
As noted earlier, biogas is often contaminated with hydrogen sulfide and other undesirable gases that must be addressed when used on an industrial scale. But the biggest hurdle to increasing use is the current flood of natural gas on the market as a result of a new drilling technique called hydraulic fracturing. This involves drilling wells and injecting under pressure a mix of water, minerals, and organic chemicals. The "fracking" mixture shatters layers of shale (a kind of rock), releasing the trapped gas. The fracking fluid often contaminates groundwater, rendering useless water wells for human consumption. The freed natural gas sometimes also enters the well water; there are houses that can ignite the water-gas mix that comes from their taps! The fracking industry is excluded from US EPA regulation by a law passed in the early years of the George W. Bush administration. Despite the problems associated with hydraulic fracturing, the practice is widespread and has flooded the market with inexpensive natural gas. This makes it more difficult for biogas to compete for market share.
Burning garbage for energy and waste elimination
Above you learned how trash can be turned into methane in landfills. Another approach is to incinerate the trash directly, and harvest the thermal energy to do work. People have used fire for years to eliminate combustible trash and yard waste. Autumn in most northern cities and towns in the United States was characterized by a dense smoky haze from burning piles of leaves in the street. The practice fell out of favor in the 1970s with the growth of environmental awareness.
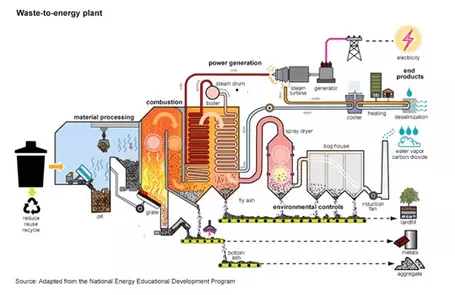
Landfills are expensive to operate, and available land is scarce. New York City closed its last landfill in 2001 and now exports all of its solid waste to places as far away as Virginia. Thus incineration of waste is an attractive alternative. When coupled with the generation of useful energy it becomes even more so.
Oslo, Norway, is a city of 1.4 million people. It has a problem — it has to import trash from neighboring Sweden to keep its garbage-to-energy plants at full capacity. The city uses incinerators to make heat for schools and to drive turbines to make electricity for the grid. The idea of burning garbage for energy is widespread in Northern Europe and Germany.
Incineration must be done carefully to avoid production of toxic gases and ash. The plants of Northern Europe are picky about what they burn. They also make sure to maintain the high temperatures needed for complete combustion. But even the cleanest burn turns everything to water and carbon dioxide, and CO2 is a greenhouse gas.
- Waste-To-Energy Plant article from ecomaine.org
- New York Times article: Oslo is short on trash to burn for energy
Bio oil and other lipids from biomass
We have explored how biomass can be turned into methane and ethanol. Another approach is to harvest high-energy lipids (oils, fats, and waxes) from plants and algae. The first uses of lipid biomass for fuel were probably the burning of plant and animal oils and fats in lamps for lighting and stoves for cooking. Oil squeezed from olives had widespread use in the Mediterranean during ancient times. Surely early humans noted the burning of fat while cooking game over an open flame. Simple oil lamps carved from stone have been dated to 15,000 years BCE, and the practice may be as ancient as 70,000 BCE.
The whaling industry grew to a worldwide scale from 1600 to 1900, largely based on the demand for high-quality oil to illuminate homes. Whale oil was made by boiling the whales blubber — a process called rendering. Whale oil was renowned for its bright flame and minimal production of soot. The demand for this product was so great that it led to the extinction of many local whale populations, and the near extinction of almost all of the species. Whales were only saved from complete extinction by an international ban on their killing in the 1960s (which is ignored by Japan and Norway). Another saving grace for whales was the development of oil wells (petroleum) during the mid 19th century. These provided kerosene, a less expensive substitute for whale oil. Note that it was the business of making oil for lamps that made John D. Rockefeller his first fortune. Only after 1900 did the market shift to production of oil for heating homes and gasoline for cars and trucks.
Whaling for bio oil to burn in lamps was lethal for whales, and many whalers.
Candles are made of wax or modified fat from animals or plants and include a wick up which the melted wax flows. Candles were used in ancient Egypt and Rome, and many other cultures. Their use dates back to at least 5,000 years BCE. In colonial America, tallow (fat from beef or other animals) was used to make candles. The fat was heated in a pot, and wicks of jute or cotton were dipped multiple times until the desired candle thickness was achieved.
Biodiesel is a fuel made from any combination of the following: plant oils, animal oils, waste cooking grease and oil. It burns in diesel engines very much like diesel fuel from petroleum and on the whole causes fewer tailpipe emissions. Crops used for biodiesel include; rapeseed (canola), palm, cotton seed, jatropha bush, soybean, sunflower seeds. Some of these oil plants are controversial. Much of the natural Indonesian forest is being replaced by palm oil trees. This is a boost for that nation's economy but an ecological calamity for the forest.
US biodiesel production has reached about 1.5 billion gallons per year for 2013, 2014, and 2015. Another 50 billion gallons of biodiesel is imported to the US market. Considering that the United States consumed about 297 billion gallons of petroleum in 2015, biodiesel accounted for only 0.7% of oil used. The production is increasing worldwide.
Another potential source of biofuel is oils harvested from microalgae. These are simple plant-like creatures that live in water and photosynthesize. Not being plants, they do not need to devote energy to non-photosynthetic structures like stems, roots, and wood. Under ideal conditions, algae can double their biomass every day. Two groups of algae are particularly promising for bio-lipid production, diatoms and blue-green algae. The latter is actually a type of bacteria called cyanophyta. Freshwater algae are easier to cultivate than saltwater varieties. Microalgae have the potential to produce a much higher yield of oil per unit area than plants.
An experimental facility in Israel uses carbon dioxide-rich flue gas from a coal-burning power plant to enrich ponds for growing algae. The algae are harvested for their oils.
Using land to produce energy from biofuels is less efficient than using photovoltaics to make electricity, but biofuels provide advantages for storage and transport
On an area use basis, photovoltaic (PV) cells will provide easily two to four times as much energy than current biofuels on the market. This traces to the relative efficiency of modern photovoltaic cells and the inefficiency of photosynthesis. High-efficiency PV arrays can turn as much as 20% of the incident sunlight into electricity, but 15% is the more typical number. Photosynthesis by plants can only capture about 8% of the energy from sunlight, and the typical number is closer to 1%. In practice one can expect PV cells to produce a minimum of 8.4 kWh per m2 of land per year. Corn-to-ethanol comes in at 2–2.5 kWh per m2 of land per year, and a better crop such as Miscanthus produces 4.6 kWh per m2 of land per year.
The biofuels have the advantage of easy use for powering cars and trucks. Current batteries on electric vehicles simply do nt provide the range of travel delivered by liquid or compressed gas fuels. However, electricity can be used to create hydrogen gas from water, and compressed hydrogen can provide the needed vehicle range. Hydrogen gas also burns completely clean, producing only water vapor and no carbon emissions at all. In practice, storing hydrogen and converting it back to electricity is at least 70% efficient.
(Source: Wikipedia article on solar cells)
Collecting your thoughts: Systems thinking and reflection
You have just learned about the history of using energy from biofuels. Take a few moments to consider how this all fits together. You learned that biofuels require different amounts of processing before they can be used. Some need only mechanical processing, and others require biological activity to make them useful. Think of an example for each.
You learned that producing ethanol from sugar or corn requires fermentation followed by the step of distillation to concentrate the alcohol. Think about the fermentation process as a system. What goes in and what comes out? What internal system control stops the production of ethanol when the brew reaches about 18% alcohol? Is that an example of a negative or a positive feedback mechanism?
Can you think of a way to use biofuel on your college campus? Think of the college campus as a system. How does energy enter that system? How does it leave? Does your campus system actually create biofuel as a waste product? In what ways?
Reference for this article
M. Khanna et al. (eds.), Handbook of Bioenergy Economics and Policy, 15. Natural Resource Management and Policy 33, DOI 10.1007/978-1-4419-0369-3_2,C _Springer Science+Business Media, LLC 2010
- Article on biodigesters for methane: Methane Digesters For Fuel Gas and Fertilizer by John Fry.
- Ancient Oil Lamps History by Noufris Papatzanakis
- 10 Top Biofuel Crops article by John Perritano, from Howstuffworks.com
- US DOE: Biodisel
- Davis et al (2012): Renewable Diesel from Algal Lipids: An Integrated Baseline for Cost, Emissions, and Resource Potential from a Harmonized Model
- Energy.gov Office of Energy Efficiency and Renewable Energy: Bioenergy
- Algal Oil Yields from Oilgae.com
- Don't Be a PV Efficiency Snob by Tom Murphy
- Cellulosic Ethanol: Pros & Cons from Green the Future
- Systems Biology for Bioenergy from US DOE Office of Science
- Switchgrass Research Group: Progress Report 2012 report from energy.gov
- Methyl Tertiary Butyl Ether (MTBE) Fact Sheet from US EPA
- Bioenergy Feedstock Information Network
- Sources: Khanna et al. (2008), Cellulosic Biofuels: Are They Economically Viable and Environmentally Sustainable? by Madhu Khanna, from Choices Magazine
- WSJ on Canadian tar-sands oil article by David Roberts and published by Grist Magazine
- Article on Hydrogen from electrolysis: Electrical Efficiency of Electrolytic Hydrogen Production by Mazloomi, et al (2012)
- Hydrogen production experiment from NMSEA.org: Electrolysis: Obtaining hydrogen from water: The Basis for a Solar-Hydrogen Economy (p. 136)
- Biodiesel Production for 2015 from Biodisel.org

![[reuse info]](/images/information_16.png)

![[creative commons]](/images/creativecommons_16.png)