Nanoscience Literature for Earth and Environmental Science

Nanoscience topics can be introduced to virtually every class in the Earth and Environmental Sciences curricula. Here is a collection of over 500 references from the literature that have been identified by experts who attended the
Goldschmidt 2017 and
NanoEarth 2018 workshops. These references are organized in topics that can readily be integrated into existing courses in Mineralogy, Petrology, Geochemistry, Hydrology, Environmental Geology, and many more. This is not meant to be a comprehensive list of resources--the small world of nanoscience turns out to be a very large field of scholarship (drawing from sister disciplines in chemistry, physics, material science, chemical and environmental engineering....) . This is a place to start where you'll find easily accessible and reliable references. Unfortunately, copyright limitations do not permit us to post copies of these articles--but you can easily find these in any research library.
For faculty, we encourage you to:
- Introduce units on nanoscience into your existing courses; the nanoscience revolution has as many applications and implications as the plate tectonics revolution 50 years ago! Nanoscience introduces some really exciting new science, and it demonstrates career pathway opportunities for students.
- Aggregate these topics into a new course on nanoscience! There is a great need for a new generation of nanoscience courses in the Earth and Environmental Sciences.
- Create new teaching activities on nanoscience: new lectures based on the literature, class demonstrations, teaching activities and problem sets, laboratory experiments, course-long projects....
Please Contribute a Resource at this online form. Please contribute: a) additional references that you use in your own teaching and research; b) course outlines and syllabi; and c) teaching activities you've developed to share with the community.
We invite your feedback: Please take a look through this collection of resources and take a minute to provide your feedback using this user survey.
Teaching Strategies for Teaching with the Primary Literature
Many of us use the primary literature in our courses. However, students (particularly undergraduates) need some guidance in learning how to mine the information in professional scientific writings. Here are a few examples of different approaches one can take in teaching students how to read journal articles. (These teaching strategies derive from the On the Cutting Edge program Teaching Petrology in the 21st Century module).
Recommended References for Teaching About Nanoscience in the Earth and Environmental Sciences
The following references have been recommended, vetted, and submitted by an international collaboration of nanoscience experts who attended the 2017 and 2018 Goldschmidt Teaching Nanoscience workshops, and the 2018 NanoEarth workshop. These references provide an excellent foundation to develop lectures, reading lists, and other class activities to introduce Nanoscience in your Earth and Environmental Science courses (e.g., Mineralogy, Petrology, Geochemistry, Hydrology, Environmental Science, ...). Please share any derivative resources by contributing to our Teaching Activity Collections Contribute a Resource (course description, teaching activity, other references or links that you use in your courses...).
Build these topics into your course lectures and instructional activities:
Overview | Nanoparticles (Nanominerals, Engineered, Incidental) | NPs in Earth System (Fluxes, rates, transport, transformation) | Characterization (analytical methods applied to NPs) | Nanoprocesses (thermodynamics/kinetics, crystallization, dissolution, catalysis, sorption, photochemical/redox, thin films) | Size Dependent Properties (optical, physical, structure) | NPs in Ocean Systems (NPs in Ocean, Fe-fertility, biogeochemistry, hydrothermal vents) | NPs in Freshwater (rivers, waste water treatment) | NPs in Atmosphere (pathways and processes, natural NPs, anthropogenic NPs) | Climate Change (carbon sequestration, coal/biomass burning, geoengineering) | NPs in Soil and Critical Zone (chemical weathering) | Natural NPs (ore bodies/mine waste, fault zones, extraterrestrial) | NPs and Life (NPs and origin of life, NP-bio interactions, bio-generated NPs) | NPs and Human Health (diagnosis, drug delivery, toxicity, NPs in food and consumer products, NP metals, NPs and respiratory disease) | NPs and Environmental Hazards (engineered NPs, heavy metals, plastics, radioactive waste, fullerenes) | NPs and Risk and Societal Issues | Ethics and Nanoscience
Nanoscience in the Earth and Environmental Sciences: An Overview (Start here)!
Start with the latest review article on nanoscience in the Earth and Environmental Sciences: Hochella, M. F., Mogk, D. W., Ranville, J., Allen, I. C., Luther, G. W., Marr, L. C., McGrail, B. P., Murayama, M., Qafoku, N. P., Rosso, K. M., Sahai, N., Schroeder, P. A., Vikesland, P., Westerhoff, P., and Yang, Y., 2019,
Natural, incidental, and engineered nanomaterials and their impacts on the Earth system: Science, v. 363, no. 6434, p. eaau8299. DOI: 10.1126/science.aau8299. This is the most comprehensive, up-to-date review of the many occurrences of nanoparticles in the Earth system, and the impacts they have on Earth processes and environmental and human health.
- Hochella, M. F., Lower, S. K., Maurice, P. A., Penn, R. L., Sahai, N., Sparks, D. L., and Twining, B. S., 2008, Nanominerals, mineral nanoparticles, and earth systems: Science, v. 319, no. 5870, p. 1631-1635.
- Hochella Jr, M. F., 2002, There's plenty of room at the bottom: Nanoscience in geochemistry: Geochimica et Cosmochimica Acta, v. 66, no. 5, p. 735-743.
- Hochella, M. F., 2002, Nanoscience and technology: the next revolution in the Earth sciences: Earth and Planetary Science Letters, v. 203, no. 2, p. 593-605.
- Hochella, M. F., 2006, The case for nanogeoscience: Annals of the New York Academy of Sciences, v. 1093, no. 1, p. 108-122.
- Hochella Jr, M., 2008, Nanogeoscience: From Origins to Cutting-Edge Applications: Elements, v. 4, no. 6, p. 373-379.
- Hochella, M., Aruguete, D., Kim, B., and Elwood Madden, A., 2012, Naturally occurring inorganic nanoparticles: general assessment and a global budget for one of earth's last unexplored major geochemical components, Pan Stanford Publishing Pte. Ltd., Nature's Nanostructures, 1-42 p.
- Hochella, M. F., Spencer, M. G., and Jones, K. L., 2015, Nanotechnology: nature's gift or scientists' brainchild?: Environmental Science: Nano, v. 2, no. 2, p. 114-119.
- Bursten, J. R., Roco, M. C., Yang, W., Zhao, Y., Chen, C., Savolainen, K., Gerber, C., Kataoka, K., Krishnan, Y., and Bayley, H., 2016, Nano on reflection: Nature Nanotechnology, v. 11, no. 10, p. 828-834.
National Nanotechnology Initiative Strategic Plan
- National Science and Technology Council, C. o. T., Subcommittee on Nanoscale Science, and Engineering, a. T., 2014, National Nanotechnology Initiative Strategic Plan p. 88.
Convergent Science
- McNutt, M. K., 2017, Convergence in the Geosciences: GeoHealth, v. 1, no. 1, p. 2-3.
- National Research Council, 2014, Convergence: Facilitating Transdisciplinary Integration of Life Sciences, Physical Sciences, Engineering, and Beyond, Washington, DC, The National Academies Press, 152 p.
Nanominerals- Mineral Nanoparticles
- Caraballo, M. A., Michel, F. M., and Hochella Jr, M. F., 2015, The rapid expansion of environmental mineralogy in unconventional ways: Beyond the accepted definition of a mineral, the latest technology, and using nature as our guide: American Mineralogist, v. 100, no. 1, p. 14-25.
- Godelitsas, A., 2017, Mineral Surface Science and Nanogeoscience: The Case of Mineral Nanoparticles, Nanominerals and Natural Nanoporous Oxide Materials: Advanced Science Letters, v. 23, no. 6, p. 5828-5830.
- Nieto, F., and Livi, K. J., 2013, Minerals at the nanoscale, The Mineralogical Society of Great Britain and Ireland.
- Plathe, K. L., von der Kammer, F., Hassellöv, M., Moore, J. N., Murayama, M., Hofmann, T., and Hochella, M. F., 2013, The role of nanominerals and mineral nanoparticles in the transport of toxic trace metals: Field-flow fractionation and analytical TEM analyses after nanoparticle isolation and density separation: Geochimica et Cosmochimica Acta, v. 102, p. 213-225.
- Ranville, J.M., in Frontiers of Nanoscience, M. L. Baalousha, J., Ed., Size Distributions, (Elsevier, 2015), vol. 8, chap. 3, pp. 91-121.
Nanoparticles-- Engineered and Incidental
- Falinski, M. M., Plata, D. L., Chopra, S. S., Theis, T. L., Gilbertson, L. M., and Zimmerman, J. B., 2018, A framework for sustainable nanomaterial selection and design based on performance, hazard, and economic considerations: Nature nanotechnology.
- Hendren, C. O., Mesnard, X., Dröge, J., and Wiesner, M. R., 2011, Estimating production data for five engineered nanomaterials as a basis for exposure assessment, ACS Publications.
- Keller, A. A., and Lazareva, A., 2013, Predicted releases of engineered nanomaterials: from global to regional to local: Environmental Science & Technology Letters, v. 1, no. 1, p. 65-70.
- Lankone, R. S., Challis, K. E., Bi, Y., Hanigan, D., Reed, R. B., Zaikova, T., Hutchison, J. E., Westerhoff, P., Ranville, J., and Fairbrother, H., 2017, Methodology for quantifying engineered nanomaterial release from diverse product matrices under outdoor weathering conditions and implications for life cycle assessment: Environmental Science: Nano, v. 4, no. 9, p. 1784-1797.
- Lead, J. R., Aruguete, D. M., and Hochella Jr, M. F., 2010, Manufactured nanoparticles in the environment: Environmental Chemistry, v. 7, no. 1, p. 1-2.
- Linsinger, T., Roebben, G., Gilliland, D., Calzolai, L., Rossi, F., Gibson, N., and Klein, C., 2012, Requirements on measurements the European Commission definition of the term "nanomaterial"
- Mueller, N. C., and Nowack, B., 2008, Exposure modeling of engineered nanoparticles in the environment: Environmental science & technology, v. 42, no. 12, p. 4447-4453.
- Niu, J., Rasmussen, P. E., Magee, R., and Nilsson, G., 2015, Spatial and temporal variability of incidental nanoparticles in indoor workplaces: impact on the characterization of point source exposures: Environ Sci Process Impacts, v. 17, no. 1, p. 98-109.
- Pati, P., McGinnis, S., and Vikesland, P. J., 2014, Life cycle assessment of "green" nanoparticle synthesis methods: Environmental Engineering Science, v. 31, no. 7, p. 410-420.
- Pavía-Sanders, A., Zhang, S., Flores, J. A., Sanders, J. E., Raymond, J. E., and Wooley, K. L., 2013, Robust magnetic/polymer hybrid nanoparticles designed for crude oil entrapment and recovery in aqueous environments: ACS nano, v. 7, no. 9, p. 7552-7561.
- Peters, T. M., Elzey, S., Johnson, R., Park, H., Grassian, V. H., Maher, T., and O'Shaughnessy, P., 2009, Airborne monitoring to distinguish engineered nanomaterials from incidental particles for environmental health and safety: J Occup Environ Hyg, v. 6, no. 2, p. 73-81.
- Petosa, A. R., Jaisi, D. P., Quevedo, I. R., Elimelech, M., and Tufenkji, N., 2010, Aggregation and deposition of engineered nanomaterials in aquatic environments: role of physicochemical interactions: Environmental science & technology, v. 44, no. 17, p. 6532-6549.
- Pokropivny, V. V., and Skorokhod, V. V., 2007, Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science: Materials Science and Engineering: C, v. 27, no. 5-8, p. 990-993.
- Riquelme, M. V., Leng, W., Carzolio, M., Pruden, A., and Vikesland, P., 2017, Stable oligonucleotide-functionalized gold nanosensors for environmental biocontaminant monitoring: Journal of Environmental Sciences, v. 62, p. 49-59.
- Simonin, M., Colman, B. P., Anderson, S. M., King, R. S., Ruis, M. T., Avellan, A., Bergemann, C. M., Perrotta, B. G., Geitner, N. K., and Ho, M., 2018, Engineered nanoparticles interact with nutrients to intensify eutrophication in a wetland ecosystem experiment: Ecological Applications, v. 28, no. 6, p. 1435-1449.
- Sun, T. Y., Gottschalk, F., Hungerbuhler, K., and Nowack, B., 2014, Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials: Environ Pollut, v. 185, p. 69-76.
- Wagner, S., Gondikas, A., Neubauer, E., Hofmann, T., and von der Kammer, F., 2014, Spot the difference: engineered and natural nanoparticles in the environment--release, behavior, and fate: Angew Chem Int Ed Engl, v. 53, no. 46, p. 12398-12419.
- Walker, W. C., Bosso, C. J., Eckelman, M., Isaacs, J. A., and Pourzahedi, L., 2015, Integrating life cycle assessment into managing potential EHS risks of engineered nanomaterials: reviewing progress to date: Journal of Nanoparticle Research, v. 17, no. 8, p. 344.
- Westerhoff, P., and Nowack, B., 2012, Searching for global descriptors of engineered nanomaterial fate and transport in the environment: Accounts of chemical research, v. 46, no. 3, p. 844-853.
- Yang, Z., Choi, D., Kerisit, S., Rosso, K. M., Wang, D., Zhang, J., Graff, G., and Liu, J., 2009, Nanostructures and lithium electrochemical reactivity of lithium titanites and titanium oxides: A review: Journal of Power Sources, v. 192, no. 2, p. 588-598.
- Zhou, J., Li, J., Du, X., and Xu, B., 2017, Supramolecular biofunctional materials: Biomaterials, v. 129, p. 1-27.
Nanoparticles in the Earth System: Fluxes, Rates, Transport, Transformation

The global budget for naturally occurring inorganic nanoparticles. All numbers are in units of terragrams (Tg = 1012 g). All italicized numbers are fluxes (Tg yr-1), and the numbers in rectangular boxes are reservoir sizes, if known. Some of the nanomaterial fluxes are listed as two components, explained as follows: for the volcanic input to the atmosphere, 20 Tg is due to SO2 aerosol formation, and 2 Tg is due to mineral ash. For the three aeolian inputs to the continents, continental shelves, and the open oceans, the first number is due to the 320 Tg continental mineral dust output, and the second number is due to the 22 Tg volcanic output.
![[creative commons]](/images/creativecommons_16.png)
Provenance: Figure from Michael Hochella, Virginia Tech, NNCI NanoEarth Project
Reuse: This item is offered under a Creative Commons Attribution-NonCommercial-ShareAlike license http://creativecommons.org/licenses/by-nc-sa/3.0/ You may reuse this item for non-commercial purposes as long as you provide attribution and offer any derivative works under a similar license.
- Banfield, J. F., and Zhang, H., 2001, Nanoparticles in the environment: Reviews in mineralogy and geochemistry, v. 44, no. 1, p. 1-58.
- Barnard, A. S., and Guo, H., 2012, Nature's Nanostructures, CRC Press.
- Batley, G. E., Kirby, J. K., and McLaughlin, M. J., 2013, Fate and Risks of Nanomaterials in Aquatic and Terrestrial Environments: Accounts of Chemical Research, v. 46, no. 3, p. 854-862.
- Bertsch, P. M., 2014, It's been nano all along!: The occurrence, behaviour, and fate of natural and manufactured nano-minerals/materials in the environment: Australian Clay Minerals Society Conference.
- Graca, B., Zgrundo, A., Zakrzewska, D., Rzodkiewicz, M., and Karczewski, J., 2018, Origin and fate of nanoparticles in marine water–Preliminary results: Chemosphere
- Hendren, C. O., Mesnard, X., Dröge, J., and Wiesner, M. R., 2011, Estimating production data for five engineered nanomaterials as a basis for exposure assessment, ACS Publications.
- Hochella, M., Aruguete, D., Kim, B., and Elwood Madden, A., 2012, Naturally occurring inorganic nanoparticles: general assessment and a global budget for one of earth's last unexplored major geochemical components, Pan Stanford Publishing Pte. Ltd., Nature's Nanostructures, 1-42 p.
- Klaine Stephen J.,Alvarez Pedro J. J., Batley Graeme E., Fernandes Teresa F. ,Handy Richard D., Lyon Delina Y., Mahendra Shaily, McLaughlin Michael J., Lead Jamie R.., 2008, Nanomaterials in the environment: Behavior, fate, bioavailability, and effects: Environmental Toxicology and Chemistry, v. 27, no. 9, p. 1825-1851.
- Lohmann, U., and Feichter, J., 2005, Global indirect aerosol effects: a review: Atmospheric Chemistry and Physics, v. 5, no. 3, p. 715-737.
- Lowry, G. V., Gregory, K. B., Apte, S. C., and Lead, J. R., 2012, Transformations of nanomaterials in the environment: Environ Sci Technol, v. 46, no. 13, p. 6893-6899.
- Lowry, G. V., Espinasse, B. P., Badireddy, A. R., Richardson, C. J., Reinsch, B. C., Bryant, L. D., Bone, A. J., Deonarine, A., Chae, S., Therezien, M., Colman, B. P., Hsu-Kim, H., Bernhardt, E. S., Matson, C. W., and Wiesner, M. R., 2012, Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large scale freshwater emergent wetland: Environ Sci Technol, v. 46, no. 13, p. 7027-7036.
- Luther, G. W., 2016, Inorganic Chemistry for Geochemistry and Environmental Sciences: Fundamentals and Applications, John Wiley & Sons.
- Miseljic, M., and Olsen, S. I., 2014, Life-cycle assessment of engineered nanomaterials: a literature review of assessment status: Journal of Nanoparticle Research, v. 16, no. 6, p. 2427.
- O'Dowd, C. D., Smith, M. H., Consterdine, I. E., and Lowe, J. A., 1997, Marine aerosol, sea-salt, and the marine sulphur cycle: A short review: Atmospheric Environment, v. 31, no. 1, p. 73-80.
- Poulton, S. W., and Raiswell, R., 2002, The low-temperature geochemical cycle of iron: from continental fluxes to marine sediment deposition: American Journal of Science, v. 302, no. 9, p. 774-805.
- Pourzahedi, L., et al., Life cycle considerations of nano-enabled agrochemicals: are today's tools up to the task? Environmental Science: Nano 5, 1057-1069 (2018).
- Poulton, S. W., and Raiswell, R., 2005, Chemical and physical characteristics of iron oxides in riverine and glacial meltwater sediments: Chemical Geology, v. 218, no. 3-4, p. 203-221
- Raiswell, R., Tranter, M., Benning, L. G., Siegert, M., De'ath, R., Huybrechts, P., and Payne, T., 2006, Contributions from glacially derived sediment to the global iron (oxyhydr)oxide cycle: Implications for iron delivery to the oceans: Geochimica et Cosmochimica Acta, v. 70, no. 11, p. 2765-2780.
- Rochman, C. M., 2018, Microplastics research—from sink to source: Science, v. 360, no. 6384, p. 28-29.
- Sharma, V. K., Filip, J., Zboril, R., and Varma, R. S., 2015, Natural inorganic nanoparticles--formation, fate, and toxicity in the environment: Chem Soc Rev, v. 44, no. 23, p. 8410-8423.
- Wagner, S., Gondikas, A., Neubauer, E., Hofmann, T., and von der Kammer, F., 2014, Spot the difference: engineered and natural nanoparticles in the environment--release, behavior, and fate: Angew Chem Int Ed Engl, v. 53, no. 46, p. 12398-12419.
- Westerhoff, P., and Nowack, B., 2012, Searching for global descriptors of engineered nanomaterial fate and transport in the environment: Accounts of chemical research, v. 46, no. 3, p. 844-853.
- Wiesner, M. R., Lowry, G. V., Casman, E., Bertsch, P. M., Matson, C. W., Di Giulio, R. T., Liu, J., and Hochella Jr, M. F., 2011, Meditations on the ubiquity and mutability of nano-sized materials in the environment: ACS Nano, v. 5, no. 11, p. 8466-8470.
- NanoFASE--Nanomaterial Fate and Speciation in the Environment; extensive website with numerous resources.
Characterization of Nanoparticles: A Sampler of Analytical Methods

Chart Comparing the Scale of Microanalytical Methods, from the Australian Mi; http://www.ammrf.org.au/myscope/analysis/introduction/croscopy and Miroanalysis Research Facility
![[creative commons]](/images/creativecommons_16.png)
Provenance: Australian Mi; http://www.ammrf.org.au/myscope/analysis/introduction/croscopy and Miroanalysis Research Facility
Reuse: This item is offered under a Creative Commons Attribution-NonCommercial-ShareAlike license http://creativecommons.org/licenses/by-nc-sa/3.0/ You may reuse this item for non-commercial purposes as long as you provide attribution and offer any derivative works under a similar license.
- Beran, A., and Libowitzky, E., 2004, Spectroscopic methods in mineralogy, The Mineralogical Society of Great Britain and Ireland.
- Calas, G., 1988, Spectroscopic methods in mineralogy and geology: Review in Mineralogy, v. 18.
- Carenco, S., Moldovan, S., Roiban, L., Florea, I., Portehault, D., Vallé, K., Belleville, P., Boissière, C., Rozes, L., and Mézailles, N., 2016, The core contribution of transmission electron microscopy to functional nanomaterials engineering: Nanoscale, v. 8, no. 3, p. 1260-1279.
- Chan, M. Y., Leng, W., and Vikesland, P. J., 2018, Surface‐Enhanced Raman Spectroscopy Characterization of Salt‐Induced Aggregation of Gold Nanoparticles: ChemPhysChem, v. 19, no. 1, p. 24-28.
- de Jonge, Niels and Ross, Francis, M., 2011, Electron microscopy of specimens in liquid, Nature Nanotechnology, v6: 695-704.
- Echigo, T., Monsegue, N., Aruguete, D. M., Murayama, M., and Hochella Jr, M. F., 2013, Nanopores in hematite (α-Fe2O3) nanocrystals observed by electron tomography: American Mineralogist, v. 98, no. 1, p. 154-162 AND Echigo, T., Aruguete, D. M., Murayama, M., and Hochella Jr, M. F., 2012, Influence of size, morphology, surface structure, and aggregation state on reductive dissolution of hematite nanoparticles with ascorbic acid: Geochimica et Cosmochimica Acta, v. 90, p. 149-162.--real time resolved dissolution experiments
- Elmi et al., 2016, Surface Crystal Chemistry of Phyllosilicates Using X-ray Photoelectron Spectroscopy: A Review, Clays and Clay Minerals, Vol. 64, No. 5, 537–551, 2016.
- Halvorson and Vikesland, 2010,Surface-Enhanced Raman Spectroscopy (SERS) for Environmental Analyses, Environ. Sci. Technol. 2010, 44, 7749–7755
- Hata, S., Miyazaki, S., Gondo, T., Kawamoto, K., Horii, N., Sato, K., Furukawa, H., Kudo, H., Miyazaki, H., and Murayama, M., 2017, In-situ straining and time-resolved electron tomography data acquisition in a transmission electron microscope: Microscopy, v. 66, no. 2, p. 143-153.
- Henderson, G., Neuville, D., and Downs, R., 2014, Spectroscopic methods in mineralogy and material sciences, Walter de Gruyter GmbH & Co KG.
- Hinton, R. W., 1995, Ion microprobe analysis in geology, Microprobe Techniques in the Earth Sciences, Springer, p. 235-289.
- Hochella, M. F., Harris, D. W., and Turner, A. M., 1986, Scanning Auger microscopy as a high-resolution microprobe for geologic materials: American Mineralogist, v. 71, no. 9-10, p. 1247-1257.
- Hochella Jr, M., 1988, Auger electron and X-ray photoelectron spectroscopies: Reviews in Mineralogy and Geochemistry, v. 18, no. 1, p. 573-637.
- Hochella Jr, M., 1990, Atomic structure, microtopography, composition, and reactivity of mineral surfaces: Reviews in Mineralogy and Geochemistry, v. 23, no. 1, p. 87-132.
- Johnston, C. T., 2010, Probing the nanoscale architecture of clay minerals: Clay Minerals, v. 45, no. 3, p. 245-279.
- Jordan, G., 2010, Nanoscopic Approaches in Earth and Planetary Sciences, The Mineralogical Society of Great Britain and Ireland.
- Klein, K.L., I.M. Anderson, N. De Jonge, 2011, Transmission electron microscopy with a liquid flow cell, Journal of Microscopy, Vol. 242, Pt 2, pp. 117–123.
- Laborda, F., Bolea, E., Cepriá, G., Gómez, M. T., Jiménez, M. S., Pérez-Arantegui, J., and Castillo, J. R., 2016, Detection, characterization and quantification of inorganic engineered nanomaterials: a review of techniques and methodological approaches for the analysis of complex samples: Analytica chimica acta, v. 904, p. 10-32.
- Lahr, R. H., and Vikesland, P. J., 2014, Surface-enhanced Raman spectroscopy (SERS) cellular imaging of intracellulary biosynthesized gold nanoparticles: ACS Sustainable Chemistry & Engineering, v. 2, no. 7, p. 1599-1608.
- Lee, et al., 2014, Nanoparticle Size Detection Limits by Single Particle ICP-MS for 40 Elements, Environ. Sci. Technol. 48, 10291 − 10300
- Linsinger T., Roebben G., Gilliland D., Calzolai L., Rossi F., Gibson N., Klein C., 2012, Requirements on measurements for the implementation of the European Commission definition of the term Nanomaterial, JRC Reference Report
- Liu, Juan, et al., 2009, Influence of Size and Aggregation on the Reactivity of an Environmentally and Industrially Relevant Nanomaterial (PbS), Environ. Sci. Technol. 2009, 43, 8178–8183
- McMillan, P., and Hofmeister, A., 1988, Spectroscopic methods in mineralogy and geology: Reviews in Mineralogy, v. 18, p. 99-150.
- Michen, Benjamin , Christoph Geers, Dimitri Vanhecke, Carola Endes, Barbara Rothen-Rutishauser , Sandor Balog, Alke Petri-Fink, 2015, Avoiding drying-artifacts in transmission electron microscopy: Characterizing the size and colloidal state of nanoparticles, Scientific Reports, 5: 9793
- Mogk, D. W., and Locke Iii, W. W., 1988, Application of auger electron spectroscopy (AES) to naturally weathered hornblende: Geochimica et Cosmochimica Acta, v. 52, no. 10, p. 2537-2542.
- Mogk, D. W., 1990, Application of Auger electron spectroscopy to studies of chemical weathering: Reviews of Geophysics, v. 28, no. 4, p. 337-356.
- Mogk, D. W., and Mathez, E. A., 2000, Carbonaceous films in midcrustal rocks from the KTB borehole, Germany, as characterized by time‐of‐flight secondary ion mass spectrometry: Geochemistry, Geophysics, Geosystems, v. 1, no. 11.
- Montaño, Manuel D., John W. Olesik, Angela G. Barber, Katie Challis & James F. Ranville, Single Particle ICP-MS: Advances toward routine analysis of nanomaterials, Anal Bioanal Chem (2016) 408:5053–5074
- Moore, D., 1997, Identification of clay minerals and associated minerals: X-Ray Diffraction and Analysis of Clay Minerals, p. 227-260.
- Mueller, C. W., Weber, P. K., Kilburn, M. R., Hoeschen, C., Kleber, M., and Pett-Ridge, J., 2013, Advances in the analysis of biogeochemical interfaces: NanoSIMS to investigate soil microenvironments, Advances in agronomy, Volume 121, Elsevier, p. 1-46.
- Muir, I. J., Bancroft, G. M., Shotyk, W., and Nesbitt, H. W., 1990, A SIMS and XPS study of dissolving plagioclase: Geochimica et Cosmochimica Acta, v. 54, no. 8, p. 2247-2256.
- Mullaugh, K. M., and Luther, G. W., 3rd, 2010, Spectroscopic determination of the size of cadmium sulfide nanoparticles formed under environmentally relevant conditions: J Environ Monit, v. 12, no. 4, p. 890-897.
- Murr, L. E., and Bang, J. J., 2003, Electron microscope comparisons of fine and ultra-fine carbonaceous and non-carbonaceous, airborne particulates: Atmospheric Environment, v. 37, no. 34, p. 4795-4806.
- Nielsen, Michael H., Shaul Aloni, James J. De Yoreo, 2014, In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways, Science, V. 345, n 6201, p 1158-1162.
- Oleshko, Vladimir P., Mitsuhiro Murayama, and James M. Howe, 2002, Use of plasmon spectroscopy to evaluate the mechanical properties of materials at the nanoscale, Microsc. Microanal. 8, 350–364, DOI: 10.1017.S1431927602020299
- Pams, G. A., 1989, Mineralogy in two dimensions: Scanning tunneling microscopy of semiconducting minerals with implications for geochemical reactivity: American Mineralogist, v. 74, p. 1233-1246.
- Piazolo, Sandra, Alexandre La Fontaine, Patrick Trimby, Simon Harley, Limei Yang, Richard Armstrong, Julie M. Cairney, 2015, Deformation-induced trace element redistribution in zircon revealed using atom probe tomography, Nature Communications, 7:10490
- Plathe, K. L., von der Kammer, F., Hassellöv, M., Moore, J. N., Murayama, M., Hofmann, T., and Hochella, M. F., 2013, The role of nanominerals and mineral nanoparticles in the transport of toxic trace metals: Field-flow fractionation and analytical TEM analyses after nanoparticle isolation and density separation: Geochimica et Cosmochimica Acta, v. 102, p. 213-225.
- Praetorius, A., Gundlach-Graham, A., Goldberg, E., Fabienke, W., Navratilova, J., Gondikas, A., Kaegi, R., Günther, D., Hofmann, T., and von der Kammer, F., 2017, Single-particle multi-element fingerprinting (spMEF) using inductively-coupled plasma time-of-flight mass spectrometry (ICP-TOFMS) to identify engineered nanoparticles against the elevated natural background in soils: Environmental Science: Nano, v. 4, no. 2, p. 307-314.
- Roberts, S., and Beattie, I., 1995, Micro-Raman spectroscopy in the Earth sciences, Microprobe techniques in the Earth sciences, Springer, p. 387-408.
- Shotyk, W., and Metson, J. B., 1994, Secondary ion mass spectrometry (SIMS) and its application to chemical weathering: Reviews of Geophysics, v. 32, no. 2, p. 197-220.
- Stadermann, F. J., Floss, C., Bose, M., and Lea, A. S., 2009, The use of Auger spectroscopy for the in situ elemental characterization of sub‐micrometer presolar grains: Meteoritics & Planetary Science, v. 44, no. 7, p. 1033-1049.
- Stipp, S. L., and Hochella, M. F., 1991, Structure and bonding environments at the calcite surface as observed with X-ray photoelectron spectroscopy (XPS) and low energy electron diffraction (LEED): Geochimica et Cosmochimica Acta, v. 55, no. 6, p. 1723-1736.
- Stipp, S. L., Hochella, M. F., Parks, G. A., and Leckie, J. O., 1992, Cd2+ uptake by calcite, solid-state diffusion, and the formation of solid-solution: Interface processes observed with near-surface sensitive techniques (XPS, LEED, and AES): Geochimica et Cosmochimica Acta, v. 56, no. 5, p. 1941-1954.
- Takahashi, H., McSwiggen, P., and Nielsen, C., 2014, A unique wavelength-dispersive soft X-ray emission spectrometer for electron probe X-ray microanalyzers: Microsc Anal, v. 15, p. S5-S8.
- Takahashi, H., Murano, T., Takakura, M., Asahina, S., Terauchi, M., Koike, M., Imazono, T., Koeda, M., and Nagano, T., 2016, Development of soft X-ray emission spectrometer for EPMA/SEM and its application: IOP Conference Series: Materials Science and Engineering, v. 109, no. 1, p. 012017.
- Valley, John W., Aaron J. Cavosie, Takayuki Ushikubo, David A. Reinhard, Daniel F. Lawrence, David J. Larson, Peter H. Clifton, Thomas F. Kelly, Simon A. Wilde, Desmond E. Moser, and Michael J. Spicuzza, 2014, Hadean age for a post-magma-ocean zircon confirmed by atom-probe tomography, Nature Geoscience, 7: 219-223
- Woehl, T. J., Jungjohann, K. L., Evans, J. E., Arslan, I., Ristenpart, W. D., and Browning, N. D., 2013, Experimental procedures to mitigate electron beam induced artifacts during in situ fluid imaging of nanomaterials: Ultramicroscopy, v. 127, p. 53-63.
- Yuk, J. M., Park, J., Ercius, P., Kim, K., Hellebusch, D. J., Crommie, M. F., Lee, J. Y., Zettl, A., and Alivisatos, A. P., 2012, High-resolution EM of colloidal nanocrystal growth using graphene liquid cells: Science, v. 336, no. 6077, p. 61-64.
Books on Analytical Methods Used in Nanoscience
- Banfield, J. F., and Navrotsky, A., (eds) 2001), Nanoparticles and the Environment, Reviews in Mineralogy and Geochemistry, Volume 44
- Beran, A., and Libowitzky, E., (eds), 2004, Spectroscopic Methods in Mineralogy, European Mineralogical Union EMU Notes in Mineralogy Volume 6.
- Bish, D.L., and Post, J. E., (eds), 1989, Modern Powder Diffraction, Reviews in Mineralogy Volume 20
- Brenker, F. E., and Jordan G., (eds), 2010, Nanoscopic Approaches in Earth and Planetary Sciences, European Mineralogical Union Notes in Mineralogy #8.
- Hawthorne, F. C., (ed), 1988, Spectroscopic Methods in Mineralogy and Geology, Reviews in Mineralogy Volume 18.
- Henderson, FG. F., Neuville, D. R., and Downs, R. T., (eds), 2014, Spectroscopic Methods in Mineralogy and Material Sciences, Reviews in Mineralogy and Geochemistry Volume 78
- Moore, D.M., and Reynolds, R. C., Jr., 1997, X-ray Diffraction and the Identification of Clay Minerals, Oxford Press 2nd edition.
- Nieto, F., and Livi, K. J.R., (eds), 2013, Minerals at the Nanoscale, European Mineralogical Union Notes in Mineralogy #14.
- Potts, P. J., Bowles, J.F.W., TRererd, S.J.B., ad Cave, M. R., (eds), 1995, Microprobe Techniques in the Earth Sciences. Chapman Hall, The Mineralogical Society Series #6.
- Vaughn, D. J., and Pattrick, R.A.D., (eds), 1995, Mineral Surfaces, Chapman Hall, The Mineralogical Society Series #5.
Nanoparticles and Surface Thermodynamics/Kinetics

- J., I., 1992, Intermolecular and Surface Forces, London, Academic Press.
- Lee, S., and Xu, H., 2016, Size-dependent phase map and phase transformation kinetics for nanometric iron (III) oxides (γ→ ε→ α pathway): The Journal of Physical Chemistry C, v. 120, no. 24, p. 13316-13322.
- McHale, J. M., Auroux, A., Perrotta, A. J., and Navrotsky, A., 1997, Surface energies and thermodynamic phase stability in nanocrystalline aluminas: Science, v. 277, no. 5327, p. 788-791.
- McGrail, B. P., Thallapally, P. K., Blanchard, J., Nune, S. K., Jenks, J. J., and Dang, L. X., 2013, Metal-organic heat carrier nanofluids: Nano Energy, v. 2, no. 5, p. 845-855.
- Mullaugh, K. M., and Luther, G. W., 2010, Growth kinetics and long-term stability of CdS nanoparticles in aqueous solution under ambient conditions: Journal of Nanoparticle Research, v. 13, no. 1, p. 393-404.
- Navrotsky, A., 2003, Energetics of nanoparticle oxides: interplay between surface energy and polymorphism: Geochemical Transactions, v. 4, no. 6, p. 34-37.
- Navrotsky, A., 2004, Energetic clues to pathways to biomineralization: Precursors, clusters, and nanoparticles: Proceedings of the National Academy of Sciences of the United States of America, v. 101, no. 33, p. 12096-12101.
- Navrotsky, A., 2007, Calorimetry of nanoparticles, surfaces, interfaces, thin films, and multilayers: The Journal of Chemical Thermodynamics, v. 39, no. 1, p. 1-9.
- Navrotsky, A., Mazeina, L., and Majzlan, J., 2008, Size-driven structural and thermodynamic complexity in iron oxides: Science, v. 319, no. 5870, p. 1635-1638.
- Navrotsky, A., Ma, C., Lilova, K., and Birkner, N., 2010, Nanophase transition metal oxides show large thermodynamically driven shifts in oxidation-reduction equilibria: science, v. 330, no. 6001, p. 199-201.
- Navrotsky, A., 2011, Nanoscale effects on thermodynamics and phase equilibria in oxide systems: ChemPhysChem, v. 12, no. 12, p. 2207-2215.
- Shen, Z., Chun, J., Rosso, K. M., and Mundy, C. J., 2018, Surface Chemistry Affects the Efficacy of the Hydration Force between Two ZnO (101 ̅0) Surfaces: The Journal of Physical Chemistry C, v. 122, no. 23, p. 12259-12266.
- Teng, H. H., Dove, P. M., Orme, C. A., and De Yoreo, J. J., 1998, Thermodynamics of calcite growth: baseline for understanding biomineral formation: Science, v. 282, no. 5389, p. 724-727.
- Wang, L., Ruiz-Agudo, E. n., Putnis, C. V., Menneken, M., and Putnis, A., 2011, Kinetics of calcium phosphate nucleation and growth on calcite: Implications for predicting the fate of dissolved phosphate species in alkaline soils: Environmental science & technology, v. 46, no. 2, p. 834-842.
- Williams, E. D., and Bartelt, N. C., 1991, Thermodynamics of surface morphology: Science, v. 251, no. 4992, p. 393-400.
- Zhang, H., Gilbert, B., Huang, F., and Banfield, J. F., 2003, Water-driven structure transformation in nanoparticles at room temperature: Nature, v. 424, no. 6952, p. 1025.
- Zhang, H., and Banfield, J. F., 2005, Size Dependence of the Kinetic Rate Constant for Phase Transformation in TiO2 Nanoparticles: Chemistry of Materials, v. 17, no. 13, p. 3421-3425.
- Zhang, H., Chen, B., and Banfield, J. F., 2009, The size dependence of the surface free energy of titania nanocrystals: Physical Chemistry Chemical Physics, v. 11, no. 14, p. 2553-2558.
- Zhang, H., and Banfield, J. F., 2012, Energy calculations predict nanoparticle attachment orientations and asymmetric crystal formation: The Journal of Physical Chemistry Letters, v. 3, no. 19, p. 2882-2886.
- Zhang, H., and Banfield, J. F., 2014, Interatomic Coulombic interactions as the driving force for oriented attachment: CrystEngComm, v. 16, no. 8, p. 1568-1578.
Nanomaterials and Crystallization: New Understanding of Processes and Pathways
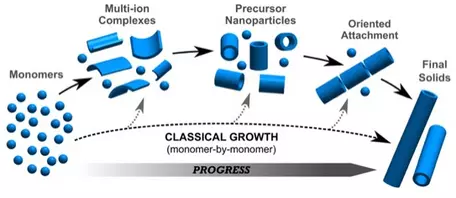
Growth of crystals from aggregated nanoparticles.
![[creative commons]](/images/creativecommons_16.png)
Provenance: Image by Dr. Marc Michel, Virginia Tech, NanoEarth NNCI project.
Reuse: This item is offered under a Creative Commons Attribution-NonCommercial-ShareAlike license http://creativecommons.org/licenses/by-nc-sa/3.0/ You may reuse this item for non-commercial purposes as long as you provide attribution and offer any derivative works under a similar license.
Classic theory of nucleation and growth of crystals assumes that crystals grow by ordering atoms (monomers) one at at a time in prescribed positions in the crystal structure. However, modern studies of growth mechanisms of crystals shows that crystals more typically grow by aggregation of nanoparticles. Crystallization pathways may involve formation of multi-ion complexes from dissociated ions, to organization of these complexes into isolated nanoparticles with very short range order, to oriented aggregates of nanoparticles, and ultimate formation of macroscopic crystals. Examples of this type of crystallization pathway of crystals forming from aggregates of nanoparticles can be found in calcium carbonates, calcium phosphates, ferric hydroxides and hydroxyl-sulfates, and aluminosilicate nanoparticles. For a more detailed description of crystallization by particle attachment (CPA), see DeYoreo et al., 2015,
Crystallization by particle attachment in synthetic, biogenic, and geologic environments, Science, vol 349, issue 6247, aaa6760-1.

Classical v Non-Classical Crystal Growth
![[creative commons]](/images/creativecommons_16.png)
Provenance: Image from Dr. Manuel Caraballo, University of Chile
Reuse: This item is offered under a Creative Commons Attribution-NonCommercial-ShareAlike license http://creativecommons.org/licenses/by-nc-sa/3.0/ You may reuse this item for non-commercial purposes as long as you provide attribution and offer any derivative works under a similar license.
- See the Powerpoint presentation by Dr. Manual Caraballo, University of Chile, Crossroads in analytical chemistry and non-classical crystal nucleation: deciphering the role of inorganic polymers in poorly crystalline nanominerals nucleation presented at the 2017 Goldschmidt Nanoscience Workshop.
- Nanocrystal growth via oriented attachment--A special volume of CrystEngComm, 2014,16, edited by Hengzhong Zhang, R. Lee Penn, Zhang Lin and Helmut Colfen.
- Bots, P., Benning, L. G., Rodriguez-Blanco, J.-D., Roncal-Herrero, T., and Shaw, S., 2012, Mechanistic Insights into the Crystallization of Amorphous Calcium Carbonate (ACC): Crystal Growth & Design, v. 12, no. 7, p. 3806-3814.
- Caraballo, M. A., Michel, F. M., and Hochella Jr, M. F., 2015, The rapid expansion of environmental mineralogy in unconventional ways: Beyond the accepted definition of a mineral, the latest technology, and using nature as our guide: American Mineralogist, v. 100, no. 1, p. 14-25. "
Environmental mineralogy is rapidly expanding in technological directions that allow for the detection, characterization, and understanding of non-crystalline and poorly crystalline phases, crystalline-amorphous mixed phases, and nanosized naturally occurring materials. Specifically, this article provides a perspective view of the broad range of structural complexity/heterogeneity observed in environmental minerals and amorphous materials, as well as our current understanding of how these materials can be best observed, evaluated, and described, and why this is important in the mineralogical sciences."
- DeYoreo et al., 2015, Crystallization by particle attachment in synthetic, biogenic, and geologic environments, Science, vol 349, issue 6247, aaa6760-1.
- Dove, P. M., and Hochella, M. F., 1993, Calcite precipitation mechanisms and inhibition by orthophosphate: In situ observations by Scanning Force Microscopy: Geochimica et Cosmochimica Acta, v. 57, no. 3, p. 705-714.
- Frandsen, C., Legg, B. A., Comolli, L. R., Zhang, H., Gilbert, B., Johnson, E., and Banfield, J. F., 2014, Aggregation-induced growth and transformation of β-FeOOH nanorods to micron-sized α-Fe 2 O 3 spindles: CrystEngComm, v. 16, no. 8, p. 1451-1458.
- Hövelmann, J. r., and Putnis, C. V., 2016, In situ nanoscale imaging of struvite formation during the dissolution of natural brucite: implications for phosphorus recovery from wastewaters: Environmental science & technology, v. 50, no. 23, p. 13032-13041.
- Hufschmid, R., Newcomb, C. J., Grate, J. W., De Yoreo, J. J., Browning, N. D., and Qafoku, N. P., 2017, Direct Visualization of Aggregate Morphology and Dynamics in a Model Soil Organic–Mineral System: Environmental Science & Technology Letters, v. 4, no. 5, p. 186-191.
- Jones, F., and Ogden, M. I., 2010, Controlling crystal growth with modifiers: CrystEngComm, v. 12, no. 4, p. 1016-1023.
- Kulmala, M., 2003, How particles nucleate and grow: Science, v. 302, no. 5647, p. 1000-1001.
- Lee, S., and Xu, H., 2016, Size-dependent phase map and phase transformation kinetics for nanometric iron (III) oxides (γ→ ε→ α pathway): The Journal of Physical Chemistry C, v. 120, no. 24, p. 13316-13322.
- Li, D., Nielsen, M. H., Lee, J. R. I., Frandsen, C., Banfield, J. F., and De Yoreo, J. J., 2012, Direction-specific interactions control crystal growth by oriented attachment: Science, v. 336, no. 6084, p. 1014-1018.
- Luther III, G. W., Theberge, S. M., and Rickard, D. T., 1999, Evidence for aqueous clusters as intermediates during zinc sulfide formation: Geochimica et Cosmochimica Acta, v. 63, no. 19-20, p. 3159-3169.
- Mashal, K., Harsh, J. B., Flury, M., and Felmy, A. R., 2005, Analysis of precipitates from reactions of hyperalkaline solutions with soluble silica: Applied Geochemistry, v. 20, no. 7, p. 1357-1367.
- Nielsen, M. H., Aloni, S., and De Yoreo, J. J., 2014, In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways: Science, v. 345, no. 6201, p. 1158-1162.
- Penn, R. L., and Banfield, J. F., 1998, Imperfect Oriented Attachment: Dislocation Generation in Defect-Free Nanocrystals: Science, v. 281, no. 5379, p. 969-971.
- Penn, R. L., and Banfield, J. F., 1998, Oriented attachment and growth, twinning, polytypism, and formation of metastable phases: Insights from nanocrystalline TiO2: American Mineralogist, v. 83, no. 9-10, p. 1077-1082.
- Penn, R. L., and Soltis, J. A., 2014, Characterizing crystal growth by oriented aggregation: CrystEngComm, v. 16, no. 8, p. 1409-1418.
- Raju, M., Van Duin, A. C. T., and Fichthorn, K. A., 2014, Mechanisms of oriented attachment of TiO2 nanocrystals in vacuum and humid environments: reactive molecular dynamics: Nano letters, v. 14, no. 4, p. 1836-1842.
- Rodriguez-Blanco, J. D., Shaw, S., and Benning, L. G., 2011, The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite: Nanoscale, v. 3, no. 1, p. 265-271.
- Rodriguez-Navarro, C., Burgos Cara, A., Elert, K., Putnis, C. V., and Ruiz-Agudo, E., 2016, Direct nanoscale imaging reveals the growth of calcite crystals via amorphous nanoparticles: Crystal Growth & Design, v. 16, no. 4, p. 1850-1860.
- Rozan, T. F., Lassman, M. E., Ridge, D. P., and Luther III, G. W., 2000, Evidence for iron, copper and zinc complexation as multinuclear sulphide clusters in oxic rivers: Nature, v. 406, no. 6798, p. 879.
- Rozan, T. F., and Luther III, G. W., 2002, Voltammetric evidence suggesting Ag speciation is dominated by sulfide complexation in river water, ACS Publications.
- Rozan, T. F., Luther, G. W., Ridge, D., and Robinson, S., 2003, Determination of Pb complexation in oxic and sulfidic waters using pseudovoltammetry: Environmental science & technology, v. 37, no. 17, p. 3845-3852.
- Shen, S., Tang, Z., Liu, Q., and Wang, X., 2010, Precisely controlled growth of heterostructured nanocrystals via a dissolution-attachment process: Inorg Chem, v. 49, no. 17, p. 7799-7807.
- Song, R. Q., and Cölfen, H., 2010, Mesocrystals—Ordered nanoparticle superstructures: Advanced materials, v. 22, no. 12, p. 1301-1330. "Mesocrystals are 3D ordered nanoparticle superstructures, often with internal porosity, which receive much recent research interest. While more and more mesocrystal systems are found in biomineralization or synthesized, their potential as material still needs to be explored...This shows the importance of mesocrystals not only for the field of materials research and allows the appliction of mesocrystals in advanced materials synthesis or property improvement of existing materials."
- Stöber, W., Fink, A., and Bohn, E., 1968, Controlled growth of monodisperse silica spheres in the micron size range: Journal of colloid and interface science, v. 26, no. 1, p. 62-69.
- Sushko, M. L., and Rosso, K. M., 2016, The origin of facet selectivity and alignment in anatase TiO2 nanoparticles in electrolyte solutions: implications for oriented attachment in metal oxides: Nanoscale, v. 8, no. 47, p. 19714-19725.
- Van Driessche, A. E. S., Benning, L. G., Rodriguez-Blanco, J. D., Ossorio, M., Bots, P., and García-Ruiz, J. M., 2012, The role and implications of bassanite as a stable precursor phase to gypsum precipitation: science, v. 336, no. 6077, p. 69-72. "Calcium sulfate minerals such as gypsum play important roles in natural and industrial processes, but their precipitation mechanisms remain largely unexplored. We used time-resolved sample quenching and high-resolution microscopy to demonstrate that gypsum forms via a three-stage process: (i) homogeneous precipitation of nanocrystalline hemihydrate bassanite below its predicted solubility, (ii) self-assembly of bassanite into elongated aggregates co-oriented along their c axis, and (iii) transformation into dihydrate gypsum. These findings indicate that a stable nanocrystalline precursor phase can form below its bulk solubility and that in the CaSO4 system, the self-assembly of nanoparticles plays a crucial role. Understanding why bassanite forms prior to gypsum can lead to more efficient anti-scaling strategies for water desalination and may help to explain the persistence of CaSO4 phases in regions of low water activity on Mars".
- Van Driessche, A. E. S., Stawski, T. M., Benning, L. G., and Kellermeier, M., 2017, Calcium Sulfate Precipitation Throughout Its Phase Diagram, in Van Driessche, A. E. S., Kellermeier, M., Benning, L. G., and Gebauer, D., eds., New Perspectives on Mineral Nucleation and Growth: From Solution Precursors to Solid Materials: Cham, Springer International Publishing, p. 227-256.
- Vikesland, P. J., Rebodos, R. L., Bottero, J. Y., Rose, J., and Masion, A., 2016, Aggregation and sedimentation of magnetite nanoparticle clusters: Environmental Science: Nano, v. 3, no. 3, p. 567-577.
- Vindedahl, A. M., Strehlau, J. H., Arnold, W. A., and Penn, R. L., 2016, Organic matter and iron oxide nanoparticles: aggregation, interactions, and reactivity: Environmental Science: Nano, v. 3, no. 3, p. 494-505.
- Wang, D., and Fernandez-Martinez, A., 2012, Order from disorder: Science, v. 337, no. 6096, p. 812-813. "L. Wang et al. (1) challenge our understanding of the inherent disorder that can be present in a crystal by presenting evidence for a crystalline material composed of amorphous clusters".
- Wang, L., Li, S., Ruiz-Agudo, E., Putnis, C. V., and Putnis, A., 2012, Posner's cluster revisited: direct imaging of nucleation and growth of nanoscale calcium phosphate clusters at the calcite-water interface: CrystEngComm, v. 14, no. 19, p. 6252-6256.
- Wang, L., Ruiz-Agudo, E. n., Putnis, C. V., Menneken, M., and Putnis, A., 2011, Kinetics of calcium phosphate nucleation and growth on calcite: Implications for predicting the fate of dissolved phosphate species in alkaline soils: Environmental science & technology, v. 46, no. 2, p. 834-842.
- Xue, X., Penn, R. L., Leite, E. R., Huang, F., and Lin, Z., 2014, Crystal growth by oriented attachment: kinetic models and control factors: CrystEngComm, v. 16, no. 8, p. 1419-1429.
- Yuk, J. M., Park, J., Ercius, P., Kim, K., Hellebusch, D. J., Crommie, M. F., Lee, J. Y., Zettl, A., and Alivisatos, A. P., 2012, High-resolution EM of colloidal nanocrystal growth using graphene liquid cells: Science, v. 336, no. 6077, p. 61-64.
- Yuwono, V. M., Burrows, N. D., Soltis, J. A., and Penn, R. L., 2010, Oriented Aggregation: Formation and Transformation of Mesocrystal Intermediates Revealed: Journal of the American Chemical Society, v. 132, no. 7, p. 2163-2165.
- Zhang, H., and Banfield, J. F., 2012, Energy calculations predict nanoparticle attachment orientations and asymmetric crystal formation: The Journal of Physical Chemistry Letters, v. 3, no. 19, p. 2882-2886.
- Zhang, H., De Yoreo, J. J., and Banfield, J. F., 2014, A Unified Description of Attachment-Based Crystal Growth: ACS Nano, v. 8, no. 7, p. 6526-6530.
- Zhang, X., Shen, Z., Liu, J., Kerisit, S. N., Bowden, M. E., Sushko, M. L., De Yoreo, J. J., and Rosso, K. M., 2017, Direction-specific interaction forces underlying zinc oxide crystal growth by oriented attachment: Nature Communications, v. 8, no. 1, p. 835.
- See the special themed volume on Nanocrystal Growth Via Oriented Attachment of CrystEngComm, 2014,vol 16, http://dx.doi.org/10.1039/C4CE90010C , and particularly articles by Penn and Soltis, Characterizing crystal growth by oriented aggregation and Zhang and Banfield Interatomic Coulombic interactions as the driving force for oriented attachment.
- The Mineral Warter Interface (Acrobat (PDF) 922kB Jun12 18) (table of contents), Elements Magazine, June 2013, volume 9 #3, (eds) Christine Putnis and Encarnacion Ruiz-Agudo.
Dissolution Reactions and Kinetics Involving Nanominerals
Size Dependent Dissolution
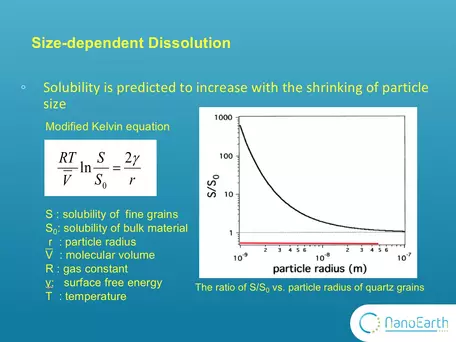
Graph showing change in dissolution properties of quartz as a function of particle size.
![[creative commons]](/images/creativecommons_16.png)
Provenance: Image provided by Michael Hochella, Virginia Tech, NanoEarth NNCI project.
Reuse: This item is offered under a Creative Commons Attribution-NonCommercial-ShareAlike license http://creativecommons.org/licenses/by-nc-sa/3.0/ You may reuse this item for non-commercial purposes as long as you provide attribution and offer any derivative works under a similar license.
One of the most important properties of nanominerals is solubility. The size effect on dissolution has long been described by this modified version of Kelvin equation.The Kelvin equation may be written in the form:
`ln(p/p_0) = (2gammaV_m)/(rRT)`
where p is the actual vapour pressure, p0 is the saturated vapour pressure, γ is the surface tension, Vm is the molar volume of the liquid, R is the universal gas constant, r is the radius of the droplet, and T is temperature. In the diagram, So is the solubility of the bulk (macro) material and can be substituted for Po. It is typically measured in conventional dissolution studies. S is the solubility of fine (nano-scale) particles and can be substituted for P. This equation indicates that, as the particle size decreases, the solubility is expected to exponentially increase. This plot shows the ratio of S to So vs particle radius for quartz grains. It was calculated according to this equation using the parameters of quartz grains. You can see that when the size is larger than 10-7 m that is 100nm, this ratio equals ~1. Nothing changes with the size. Only when the size goes down to the nanoscale, the ratio substantially deviates from 1 and the size effect on solubility can be observed. However, this equation was proposed based on theoretic calculation. Very few experimental studies have been reported to support it. The image and explanation was contributed by Michael Hochella, Virginia Tech, NanoEarth NNCI project.
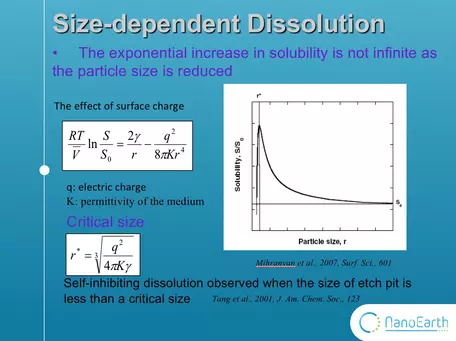
Graph showing lower limits to dissolution at the nanoscale.
![[creative commons]](/images/creativecommons_16.png)
Provenance: Figure by Michael Hochella, Virginia Tech, NanoEarth NNCI Project.
Reuse: This item is offered under a Creative Commons Attribution-NonCommercial-ShareAlike license http://creativecommons.org/licenses/by-nc-sa/3.0/ You may reuse this item for non-commercial purposes as long as you provide attribution and offer any derivative works under a similar license.
But wait! There's more! In addition, the range of validity of the modified kelvin equation has been questioned. This equation is a correction to the kelvin equation.
This added term shows the effect of surface charge. In this case,
the increase in solubility is not infinite as the size is reduced. T
here is a critical size, which is always at the nanoscale. When the size is smaller than the critical value, the solubility will decrease with the shrinking of particle size. Moreover, a recent study found that dissolution slows down or even stop at undersaturation when the size of dissolution etch pit is smaller than a critical size. However, this study was conducted on bulk mineral surface, not on nanoparticles. Therefore, the size effect on dissolution is not totally understood. Our work is the first study to really see the dissolution of nanoparticles under microscope. (Tang et al., 2001, J. Am. Chem. Soc., 123).
Hochella et al (2012) have discussed the impacts nanoparticles have on dissolution kinetics. Generally, nanoparticles will result in faster dissolution rates. However, in some cases dissolution rates can be inhibited by nanoparticles. Aggregates of nanoparticles can reduce or quench dissolution, and the moprhology of nanoparticles may also impact dissolution kinetics. Results from Putnis' lab (Wang, Klasa refs below), demonstrate that crystallization is possible on the nanoscale in under-saturated conditions, and that coupled reactions on mineral surfaces may facilitate dissolution of one phase (calcite) while simultaneously preciptating a related phase (apatite).

AFM Imamges of Calcite Precipitation and Dissolution
![[creative commons]](/images/creativecommons_16.png)
Provenance: Image from Dr. Christine Putnis, presented at the 2017 Goldschmidt Nanoscience Workshop
Reuse: This item is offered under a Creative Commons Attribution-NonCommercial-ShareAlike license http://creativecommons.org/licenses/by-nc-sa/3.0/ You may reuse this item for non-commercial purposes as long as you provide attribution and offer any derivative works under a similar license.
Here are some examples of size-dependent dissolution reactions and related kinetic control of dissolution at the nanoscale:
- Austrheim, H., Putnis, C. V., and Ruiz-Agudo, E., 2012, Direct Nanoscale Observations of CO2 Sequestration during Brucite [Mg (OH) 2] Dissolution: Environmental Science & Technology.
- Erbs, J. J., Gilbert, B., and Penn, R. L. (2008). Influence of size on reductive dissolution of six-line ferrihydrite, J. Phys. Chem. C, 112, pp. 12127–12133.
- Cwiertny, D. M., Hunter, G. J., Pettibone, J. M., Scherer, M. M., and Grassian, V. H. (2009). Surface chemistry and dissolution of alpha-FeOOH nanorods and microrods: environmental implications of size-dependent interactions with oxalate, J. Phys. Chem. C, 113, pp. 2175–2186.
- Echigo, T., Aruguete, D. M., Murayama, M., and Hochella Jr, M. F., 2012, Influence of size, morphology, surface structure, and aggregation state on reductive dissolution of hematite nanoparticles with ascorbic acid: Geochimica et Cosmochimica Acta, v. 90, p. 149-162.
- Echigo, T., Monsegue, N., Aruguete, D. M., Murayama, M., and Hochella Jr, M. F., 2013, Nanopores in hematite (α-Fe2O3) nanocrystals observed by electron tomography: American Mineralogist, v. 98, no. 1, p. 154-162 AND Echigo, T., Aruguete, D. M., Murayama, M., and Hochella Jr, M. F., 2012, Influence of size, morphology, surface structure, and aggregation state on reductive dissolution of hematite nanoparticles with ascorbic acid: Geochimica et Cosmochimica Acta, v. 90, p. 149-162.--real time resolved dissolution experiments
- Hellmann, R., Eggleston, C. M., Hochella Jr, M. F., and Crerar, D. A., 1990, The formation of leached layers on albite surfaces during dissolution under hydrothermal conditions: Geochimica et Cosmochimica Acta, v. 54, no. 5, p. 1267-1281.
- Hellmann, R., Wirth, R., Daval, D., Barnes, J.-P., Penisson, J.-M., Tisserand, D., Epicier, T., Florin, B., and Hervig, R. L., 2012, Unifying natural and laboratory chemical weathering with interfacial dissolution–reprecipitation: A study based on the nanometer-scale chemistry of fluid–silicate interfaces: Chemical Geology, v. 294, p. 203-216.
- Hochella, M. F. (2002). Nanoscience and technology the next revolution in the Earth sciences, Earth Planet. Sci. Lett., 203, pp. 593–605.
- Hochella, M., Aruguete, D., Kim, B., and Elwood Madden, A., 2012, Naturally occurring inorganic nanoparticles: general assessment and a global budget for one of earth's last unexplored major geochemical components, Pan Stanford Publishing Pte. Ltd., Nature's Nanostructures, 1-42 p.
- Hövelmann, J., Putnis, C. V., Ruiz-Agudo, E., and Austrheim, H., 2012, Direct nanoscale observations of CO2 sequestration during brucite [Mg (OH) 2] dissolution: Environmental science & technology, v. 46, no. 9, p. 5253-5260.
- Hövelmann, J. r., and Putnis, C. V., 2016, In situ nanoscale imaging of struvite formation during the dissolution of natural brucite: implications for phosphorus recovery from wastewaters: Environmental science & technology, v. 50, no. 23, p. 13032-13041.
- Inskeep, W. P., Nater, E. A., Bloom, P. R., Vandervoort, D. S., and Erich, M. S., 1991, Characterization of laboratory weathered labradorite surfaces using X-ray photoelectron spectroscopy and transmission electron microscopy: Geochimica et Cosmochimica Acta, v. 55, no. 3, p. 787-800.
- Klasa, J., Ruiz-Agudo, E., Wang, L. J., Putnis, C. V., Valsami-Jones, E., Menneken, M., and Putnis, A., 2013, An atomic force microscopy study of the dissolution of calcite in the presence of phosphate ions: Geochimica et Cosmochimica Acta, v. 117, p. 115-128.
- Lee, S., and Xu, H., 2016, Size-dependent phase map and phase transformation kinetics for nanometric iron (III) oxides (γ→ ε→ α pathway): The Journal of Physical Chemistry C, v. 120, no. 24, p. 13316-13322.
- Liu, J., Aruguete, D. A., Jinschek, J. R., Rimstidt, J. D., and Hochella, M. F. (2008). The non-oxidative dissolution of galena nanocrystals: Insights into mineral dissolution rates as a function of grain size, shape, and aggregation state, Geochim. Cosmochim. Ac., 72, pp. 5984–5996.
- Liu, J., Aruguete, D. M., Murayama, M., and Hochella Jr, M. F., 2009, Influence of size and aggregation on the reactivity of an environmentally and industrially relevant nanomaterial (PbS): Environmental science & technology, v. 43, no. 21, p. 8178-8183.
- Oldham, V. E., Mucci, A., Tebo, B. M., and Luther, G. W., 2017, Soluble Mn (III)–L complexes are abundant in oxygenated waters and stabilized by humic ligands: Geochimica et Cosmochimica Acta, v. 199, p. 238-246.
- Putnis, C. V., Renard, F. o., King, H. E., Montes-Hernandez, G., and Ruiz-Agudo, E., 2013, Sequestration of selenium on calcite surfaces revealed by nanoscale imaging: Environmental science & technology, v. 47, no. 23, p. 13469-13476.
- Putnis, C. V., and Ruiz-Agudo, E., 2013, The mineral–water interface: where minerals react with the environment: Elements, v. 9, no. 3, p. 177-182.
- Qafoku, N. P., Ainsworth, C. C., Szecsody, J. E., and Qafoku, O. S., 2003, Aluminum Effect on Dissolution and Precipitation Under Hyperalkaline Conditions: Journal of environmental quality, v. 32, no. 6, p. 2354-2363.
- Qafoku, N. P., Ainsworth, C. C., Szecsody, J. E., and Qafoku, O. S., 2004, Transport-controlled kinetics of dissolution and precipitation in the sediments under alkaline and saline conditions1: Geochimica et Cosmochimica Acta, v. 68, no. 14, p. 2981-2995.
- Qafoku, N. P., Qafoku, O., Ainsworth, C. C., Dohnalkova, A., and McKinley, S. G., 2007, Fe-solid phase transformations under highly basic conditions: Applied Geochemistry, v. 22, no. 9, p. 2054-2064.
- Qafoku, N. P., 2010, Terrestrial Nanoparticles and Their Controls on Soil-/Geo-Processes and Reactions, p. 33-91.
- Roelofs, F., and Vogelsberger, W. (2004). Dissolution kinetics of synthetic amorphous silica in biological-like media and its theoretical description, J. Phys. Chem. B, 108, pp. 11308–11316.
- Rubasinghege, G., Lentz, R. W., Park, H., Scherer, M. M., and Grassian, V. H. (2010). Nanorod dissolution quenched in the aggregated state, Langmuir, 26, pp. 1524–1527.
- Schmidt, J., and Vogelsberger, W. (2006). Dissolution kinetics of titanium dioxide nanoparticles: The observation of an unusual kinetic size effect, J. Phys. Chem. B, 110, pp. 3955–3963.
- Tang, R. K., Nancollas, G. H., and Orme, C. A. (2001). Mechanism of dissolution of sparingly soluble electrolytes, J. Am. Chem. Soc., 123, pp. 5437–5443.
- Tang, R., Wang, L., and Nancollas, G. H., 2004, Size-effects in the dissolution of hydroxyapatite: an understanding of biological demineralization: Journal of Materials Chemistry, v. 14, no. 14, p. 2341-2346.
- Tang, R. K., Wang, L. J., Orme, C. A., Bonstein, T., Bush, P. J., and Nancollas, G. H. (2004). Dissolution at the nanoscale: self-preservation of biominerals, Angewandte Chemie—International Edition, 43, pp.
2697–2701.
- Urosevic, M., Rodriguez-Navarro, C., Putnis, C. V., Cardell, C., Putnis, A., and Ruiz-Agudo, E., 2012, In situ nanoscale observations of the dissolution of dolomite cleavage surfaces: Geochimica et Cosmochimica Acta, v. 80, p. 1-13.
- Utsunomiya, S., Jensen, K. A., Keeler, G. J., and Ewing, R. C., 2004, Direct identification of trace metals in fine and ultrafine particles in the Detroit urban atmosphere: Environmental Science & Technology, v. 38, no. 8, p. 2289-2297.
- Vindedahl, A. M., Strehlau, J. H., Arnold, W. A., and Penn, R. L., 2016, Organic matter and iron oxide nanoparticles: aggregation, interactions, and reactivity: Environmental Science: Nano, v. 3, no. 3, p. 494-505.
- Wang, L., Ruiz-Agudo, E. n., Putnis, C. V., Menneken, M., and Putnis, A., 2011, Kinetics of calcium phosphate nucleation and growth on calcite: Implications for predicting the fate of dissolved phosphate species in alkaline soils: Environmental science & technology, v. 46, no. 2, p. 834-842.
- Wang, L., Li, S., Ruiz-Agudo, E., Putnis, C. V., and Putnis, A., 2012, Posner's cluster revisited: direct imaging of nucleation and growth of nanoscale calcium phosphate clusters at the calcite-water interface: CrystEngComm, v. 14, no. 19, p. 6252-6256.
- Yang, Z. H., and Xie, C. S. (2006). Zn2+ release from zinc and zinc oxide particles in simulated uterine solution, Colloids Surf. B, 47, pp. 140–145.
- Zhang, Y., Chen, Y., Westerhoff, P., Hristovski, K., and Crittenden, J. C., 2008, Stability of commercial metal oxide nanoparticles in water: Water research, v. 42, no. 8-9, p. 2204-2212.
We have a lot to learn about the solubility of particles at the nanoscale!
Catalysis by Nanominerals
- Chen, X., Liu, L., Peter, Y. Y., and Mao, S. S., 2011, Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals: Science, v. 331, no. 6018, p. 746-750.
- Fujishima, A., Zhang, X. T., and Tryk, D. A., 2008, TiO2 photocatalysis and related surface phenomena: Surface Science Reports, v. 63, no. 12, p. 515-582.
- Li, N., Bediako, D. K., Hadt, R. G., Hayes, D., Kempa, T. J., von Cube, F., Bell, D. C., Chen, L. X., and Nocera, D. G., 2017, Influence of iron doping on tetravalent nickel content in catalytic oxygen evolving films: Proceedings of the National Academy of Sciences, v. 114, no. 7, p. 1486-1491.
- Madden, A. S., and Hochella, M. F. (2005). A test of geochemical reactivity as a function of mineral size: Manganese oxidation promoted by hematite nanoparticles, Geochim. Cosmochim. Ac., 69, pp. 389–398.
- Madden, A. S., Hochella, M. F., and Luxton, T. P. (2006). Insights for size dependent reactivity of hematite nanomineral surfaces through Cu2+ sorption, Geochim. Cosmochim. Ac., 70, pp. 4095–4104.
- Rod, T. H., and Nørskov, J. K., 2002, The surface science of enzymes: Surface Science, v. 500, no. 1, p. 678-698. "...how to describe catalysis by enzymes, and in particular the analogies between enzyme catalyzed reactions and surface catalyzed reactions. We do this by discussing two concrete examples of reactions catalyzed both in nature (by enzymes) and in industrial reactors (by inorganic materials), and show that although analogies exist and the two kinds of catalyst can be described by similar tools, nature and human effort have come up with different solutions. This on the other hand implies that new and improved catalysts may be made by learning from nature".
Sorption by Nanominerals/Nano-sorption on Mineral Substrates
Sorption of metals and organic compounds is expected to have a larger effect on nanoparticles because of their greater surface area compared with macroscale materials. In addition, there appears to be a size effect of the sorptive capabilities of materials on the nanoscale: the surface charge of mineral nanoparticles may change with size and surface bonding environments of nanoparticles change as a function of size (see Hochella et al., 2012)
- Auffan, M., Rose, J., Proux, O., Borschneck, D., Masion, A., Chaurand, P., Hazemann, J. L., Chaneac, C., Jolivet, J. P., Wiesner, M. R., Van Geen, A., and Bottero, J. Y. (2008). Enhanced adsorption of arsenic onto maghemites nanoparticles: As(III) as a probe of the surface structure and heterogeneity, Langmuir, 24, pp. 3215–3222.
- Gao, Y., Wahi, R., Kan, A. T., Falkner, J. C., Colvin, V. L., and Tomson, A.B. (2004). Adsorption of cadmium on anatase nanoparticles—effect of crystal size and pH, Langmuir, 20, pp. 9585–9593.
- Giammar, D. E., Maus, C. J., and Xie, L. Y. (2007). Effects of particle size and crystalline phase on lead adsorption to titanium dioxide nanoparticles, Environ. Eng. Sci., 24, pp. 85–95.
- He, Y. T., Wan, J. M., and Tokunaga, T. (2008). Kinetic stability of hematite nanoparticles: the effect of particle sizes, J. Nanopart. Res., 10, pp. 321–332.
- Hochella, M. F., Kasama, T., Putnis, A., Putnis, C. V., and Moore, J. N. (2005). Environmentally important, poorly crystalline Fe/Mn hydrous oxides: Ferrihydrite and a possibly new vernadite-like mineral from the Clark Fork River Superfund Complex, Am. Mineral., 90, pp. 718–724.
- Jegadeesan, G., Al-Abed, S. R., Sundaram, V., Choi, H., Scheckel, K. G., and Dionysiou, D. D. (2010). Arsenic sorption on TiO2 nanoparticles: Size and crystallinity effects, Water Res., 44, pp. 965–973.
- Lee, S., Shen, Z., and Xu, H., 2016, Study on nanophase iron oxyhydroxides in freshwater ferromanganese nodules from Green Bay, Lake Michigan, with implications for the adsorption of As and heavy metals: American Mineralogist, v. 101, no. 9, p. 1986-1995.
- Liu, J., Thallapally, P. K., McGrail, B. P., Brown, D. R., and Liu, J., 2012, Progress in adsorption-based CO2 capture by metal-organic frameworks: Chem Soc Rev, v. 41, no. 6, p. 2308-2322.
- Madden, A. S., Hochella, M. F., and Luxton, T. P. (2006). Insights for size dependent reactivity of hematite nanomineral surfaces through Cu2+ sorption, Geochim. Cosmochim. Ac., 70, pp. 4095–4104.
- O'Reilly, S. E., and Hochella, M. F. (2003). Lead sorption efficiencies of natural and synthetic Mn and Fe-oxides, Geochim. Cosmochim. Ac., 67, pp. 4471–4487.
- Putnis, C. V., Renard, F. o., King, H. E., Montes-Hernandez, G., and Ruiz-Agudo, E., 2013, Sequestration of selenium on calcite surfaces revealed by nanoscale imaging: Environmental science & technology, v. 47, no. 23, p. 13469-13476.
- Waychunas, G. A., Kim, C. S., and Banfield, J. F. (2005). Nanoparticulate iron oxide minerals in soils and sediments: unique properties and contaminant scavenging mechanisms, J. Nanopart. Res., 7, pp. 409–433.
- Wersin, P., Hochella, M. F., Persson, P., Redden, G., Leckie, J. O., and Harris, D. W., 1994, Interaction between aqueous uranium (VI) and sulfide minerals: Spectroscopic evidence for sorption and reduction: Geochimica et Cosmochimica Acta, v. 58, no. 13, p. 2829-2843.
- Xu, H., Lee, S., and Xu, H., 2017, Luogufengite: A new nano-mineral of Fe2O3 polymorph with giant coercive field: American Mineralogist, v. 102, no. 4, p. 711-719.
- Yean, S., Cong, L., Yavuz, C. T., Mayo, J. T., Yu, W. W., Kan, A. T., Colvin, V. L., and Tomson, M. B. (2005). Effect of magnetite particle size on adsorption and desorption of arsenite and arsenate, J. Mater. Res., 20,pp. 3255–3264.
- Zhang, H. Z., Penn, R. L., Hamers, R. J., and Banfield, J. F. (1999).Enhanced adsorption of molecules on surfaces of nanocrystalline particles, J. Phys. Chem. B, 103, pp. 4656–4662.
Photochemical and Redox Reactions on Nanoparticles
- Chen, X., Liu, L., Peter, Y. Y., and Mao, S. S., 2011, Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals: Science, v. 331, no. 6018, p. 746-750.
- Fujishima, A., Zhang, X. T., and Tryk, D. A., 2008, TiO2 photocatalysis and related surface phenomena: Surface Science Reports, v. 63, no. 12, p. 515-582.
- Madhusudan Reddy, K., Baruwati, B., Jayalakshmi, M., Mohan Rao, M., and Manorama, S. V., 2005, S-, N- and C-doped titanium dioxide nanoparticles: Synthesis, characterization and redox charge transfer study: Journal of Solid State Chemistry, v. 178, no. 11, p. 3352-3358.
- Rubasinghege, G., and Grassian, V. H. (2009). Photochemistry of adsorbed nitrate on aluminum oxide particle surfaces, J. Phys. Chem. A, 113, pp. 7818–7825.
- Rubasinghege, G., Elzey, S., Baltrusaitis, J., Jayaweera, P. M., and Grassian, V. H. (2010). Reactions on atmospheric dust particles: surface photochemistry and size-dependent nanoscale redox chemistry, J. Phys. Chem. Lett., 1, pp. 1729–1737.
- Schuttlefield, J., Rubasinghege, G., El-Maazawi, M., Bone, J., and Grassian, V. H. (2008). Photochemistry of adsorbed nitrate, J. Am. Chem. Soc., 130, pp. 12210–12211.
Nanominerals that Play Essential Roles (but may not be included in models of) Chemical Reactions
Nanominerals may be overlooked as phases that are involved with chemical reactions that are typically observed and analyzed on the macroscale. Or, nanominerals may be ephemeral or transient phases that are involved in step equilibria in redox reactions as is the case with green rust. In sampling natural waters, green rust may rapidly oxidize if care is not taken to carefully keep samples under reduced conditions.
- Example of a nanomineral that is present in a series of step redox reactions that oxidizes rapidly and may be missed if appropriate sampling procedures are not used: green rust.
- Johnson, C. A., Freyer, G., Fabisch, M., Caraballo, M. A., Küsel, K., and Hochella, M. F., 2014, Observations and assessment of iron oxide and green rust nanoparticles in metal-polluted mine drainage within a steep redox gradient: Environmental Chemistry, v. 11, no. 4, p. 377-391.
- Johnson, C. A., Murayama, M., Küsel, K., and Hochella Jr, M. F., 2015, Polycrystallinity of green rust minerals and their synthetic analogs: Implications for particle formation and reactivity in complex systems: American Mineralogist, v. 100, no. 10, p. 2091-2105.
- Example of a nanomineral that may be entirely missed if analytical methods (TEM) are not used to specifically identify and characterize nanominerals present: Schwertmannite (Fe3+16(OH,SO4)12-13O16 · 10-12H2O; see entry from the Mindat database) --Plays an essential role in Acid Mine Drainage (Rio Tinto, Iberian Peninsula Spain). The AMD drains to the Odiel River basin and the Tinto River and subsequently to the Gulf of Cádiz and the Atlantic Ocean. Olías et al. (2006) estimated that the Odiel and Tinto rivers transport 7922 tons/year of iron and 183 802 tons/year of sulfate, which represents 0.32% and 0.15% of the total global riverine flux of these chemical components. Furthermore, they estimated that these rivers transport 0.15%, 3.13%, and 15.1% of the global riverine flux of arsenic, copper, and zinc, respectively. A survey of 64 AMD discharges from 25 different mines in the IPB (Espana et al. 2005) determined that schwertmannite was the "most important mineral phase, both in controlling the Fe solubility at pH 2–4, and as a sorbent of the trace elements (As, Cu, Zn)," effectively assigning schwertmannitea significant part of the global cycling of these elements, remarkably, from this single region. See also French et al., (2012).
- French, R. A., Caraballo, M. A., Kim, B., Rimstidt, J. D., Murayama, M., and Hochella Jr, M. F., 2012, The enigmatic iron oxyhydroxysulfate nanomineral schwertmannite: Morphology, structure, and composition: American Mineralogist, v. 97, no. 8-9, p. 1469-1482.
- Cánovas, C. R., Olías, M., Nieto, J. M., Sarmiento, A. M., and Cerón, J. C., 2007, Hydrogeochemical characteristics of the Tinto and Odiel Rivers (SW Spain). Factors controlling metal contents: Science of the Total Environment, v. 373, no. 1, p. 363-382.
- Olías, M., Cánovas, C. R., Nieto, J. M., and Sarmiento, A. M., 2006, Evaluation of the dissolved contaminant load transported by the Tinto and Odiel rivers (South West Spain): Applied Geochemistry, v. 21, no. 10, p. 1733-1749.
- Espana, J. S., Pamo, E. L., Santofimia, E., Aduvire, O., Reyes, J., and Barettino, D., 2005, Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): geochemistry, mineralogy and environmental implications: Applied geochemistry, v. 20, no. 7, p. 1320-1356.
Nanoscale Thin Films and Coatings on Minerals
- Bigi, A., Boanini, E., Bracci, B., Facchini, A., Panzavolta, S., Segatti, F., and Sturba, L., 2005, Nanocrystalline hydroxyapatite coatings on titanium: a new fast biomimetic method: Biomaterials, v. 26, no. 19, p. 4085-4089.
- Mogk, D. W., and Mathez, E. A., 2000, Carbonaceous films in midcrustal rocks from the KTB borehole, Germany, as characterized by time‐of‐flight secondary ion mass spectrometry: Geochemistry, Geophysics, Geosystems, v. 1, no. 11.
- Mathez, E. A., and Mogk, D. M., 1998, Characterization of carbon compounds on a pyroxene surface from a gabbro xenolith in basalt by time-of-flight secondary ion mass spectrometry: American Mineralogist, v. 83, no. 7-8, p. 918-924.
- Schindler, M., M. F. Hochella Jr, Soil memory in mineral surface coatings: Environmental processes recorded at the nanoscale. Geology 43, 415-418 (2015).
- Tingle, T. N., Hochella Jr, M. F., Becker, C. H., and Malhotra, R., 1990, Organic compounds on crack surfaces in olivine from San Carlos, Arizona and Hualalai Volcano, Hawaii: Geochimica et Cosmochimica Acta, v. 54, no. 2, p. 477-485.
Size Dependent Optical Properties
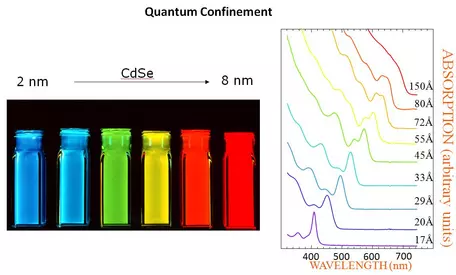
Quantum Dots as a function of size of nanoparticles. the optical absorption and emission of quantum dots shift to the blue (higher energies) as the size of the dots gets smaller. See detailed explanation by the [link http://nanocluster.mit.edu/research.php 'Bewendi Research Group'], Chemistry Dept., MIT.
![[creative commons]](/images/creativecommons_16.png)
Provenance: Bewendi Research Group'], Chemistry Dept., MIT. http://nanocluster.mit.edu/research.php
Reuse: This item is offered under a Creative Commons Attribution-NonCommercial-ShareAlike license http://creativecommons.org/licenses/by-nc-sa/3.0/ You may reuse this item for non-commercial purposes as long as you provide attribution and offer any derivative works under a similar license.
" Semiconductor nanocrystallites (quantum dots, QDs) whose radii are smaller than the bulk exciton Bohr radius constitute a class of materials intermediate between molecular and bulk forms of matter. Quantum confinement of both the electron and hole in all three dimensions leads to an increase in the effective band gap of the material with decreasing crystallite size (Figure 1). Consequently, both the optical absorption and emission of quantum dots shift to the blue (higher energies) as the size of the dots gets smaller." See the detailed description of this phenomenon from the
Bawendi Research Group at MIT.
See the online presentation on Quantum Dots by Gerhard Klimeck, posted on nanoHUB, and Amiri et al., 2013, Preparation and Optical Properties Assessment of CdSe Quantum Dots. Materials Sciences and Applications, vol 4, p. 134-137.
Gold and silver nanoparticles also show size-dependence in their optical properties. An example of changing optical properties (color) as related to nanoparticle shape (prisms v. spheres) and size can be found at Dr. Shengli Zou's (Chemistry, University of Central Florida) website on Optical properties of nanoparticles and their applications. An explanation of this phenomenon, from the nanoComposix website on Nanoparticles: Optical Properties: "Gold nanoparticles absorb and scatter light with extraordinary efficiency. Their strong interaction with light occurs because the conduction electrons on the metal surface undergo a collective oscillation when they are excited by light at specific wavelengths. This oscillation is known as a surface plasmon resonance (SPR), and it causes the absorption and scattering intensities of gold nanoparticles to be much higher than identically sized non-plasmonic nanoparticles. Gold nanoparticle absorption and scattering properties can be tuned by controlling the particle size, shape, and the local refractive index near the particle surface". An example of the application of Au nanoparticles to biomedicine can be found in the article Gold and Silver Nanoparticles: Synthesis Methods, Characterization Routes and Applications towards Drugs by Dhalid Alaquad and Tawfik Saleh, 2016, Journal of Environmental and Analytical Toxicology, 6:384. doi:10.4172/2161-0525.1000384
See also:
- Xu, H., Hill, T., Konishi, H., et al. (2017). Protoenstatite: A new mineral in Oregon sunstones with "watermelon" colors. American Mineralogist, 102(10), pp. 2146-2149. Retrieved 12 Jun. 2018, from doi:10.2138/am-2017-6186. "The crystallographically oriented nanocrystals of protoenstatite and clinoenstatite in association with copper nanocrystals are responsible for the unusual green and "watermelon" coloration of the labradorite gemstone".
Nanoparticles Affect Physical Properties--Magnetism, Mechanical Properties
- Xu, H., Lee, S., and Xu, H., 2017, Luogufengite: A new nano-mineral of Fe2O3 polymorph with giant coercive field: American Mineralogist, v. 102, no. 4, p. 711-719. "Luogufengite, Al-bearing ε-Fe2O3, is a new member of Fe2O3 polymorphs discovered in late Pleistocene basaltic scoria from the Menan Volcanic Complex nearby Rexburg, Idaho. It is an oxidation product of Fe-bearing basaltic glass at high temperature and is associated with maghemite and hematite...Luogufengite is an important mineral that records paleomagnetism of volcanic rocks because of its large magnetic coercivity. This unique magnetic property of the mineral may explain the observed unusually high-remanent magnetization in some igneous and metamorphic rocks and even martian rocks with high-remanent magnetization.
- Oleshko, V. P., Murayama, M., and Howe, J. M., 2002, Use of Plasmon Spectroscopy to Evaluate the Mechanical Properties of Materials at the Nanoscale: Microscopy and Microanalysis, v. 8, no. 4, p. 350-364.
Size-Dependent Reactivity and Structural Transformation
- Chernyshova, I., Hochella Jr, M., and Madden, A., 2007, Size-dependent structural transformations of hematite nanoparticles. 1. Phase transition: Physical Chemistry Chemical Physics, v. 9, no. 14, p. 1736-1750.
- Madden, A. S., and Hochella, M. F., 2005, A test of geochemical reactivity as a function of mineral size: Manganese oxidation promoted by hematite nanoparticles: Geochimica et Cosmochimica Acta, v. 69, no. 2, p. 389-398.
- Madden, A. S., Hochella, M. F., and Luxton, T. P., 2006, Insights for size-dependent reactivity of hematite nanomineral surfaces through Cu2+ sorption: Geochimica et Cosmochimica Acta, v. 70, no. 16, p. 4095-4104.
- Qafoku, N. P., Qafoku, O., Ainsworth, C. C., Dohnalkova, A., and McKinley, S. G., 2007, Fe-solid phase transformations under highly basic conditions: Applied Geochemistry, v. 22, no. 9, p. 2054-2064.
Nanoparticles in the Ocean/Aquatic Systems
- Blasco, J., Corsi, I., and Matranga, V., 2015, Particles in the oceans: Implication for a safe marine environment: Mar Environ Res, v. 111, p. 1-4.
- Eiriksdottir, E. S., Gislason, S. R., and Oelkers, E. H., 2015, Direct evidence of the feedback between climate and nutrient, major, and trace element transport to the oceans: Geochimica et Cosmochimica Acta, v. 166, p. 249-266.
- Findlay, A. J., Gartman, A., MacDonald, D. J., Hanson, T. E., Shaw, T. J., and Luther, G. W., 2014, Distribution and size fractionation of elemental sulfur in aqueous environments: The Chesapeake Bay and Mid-Atlantic Ridge: Geochimica et Cosmochimica Acta, v. 142, p. 334-348.
- Graca, B., Zgrundo, A., Zakrzewska, D., Rzodkiewicz, M., and Karczewski, J., 2018, Origin and fate of nanoparticles in marine water–Preliminary results: Chemosphere.
- Luther III, G. W., Meyerson, A. L., Krajewski, J. J., and Hires, R., 1980, Metal sulfides in estuarine sediments: Journal of Sedimentary Research, v. 50, no. 4.
- Luther III, G. W., Wilk, Z., Ryans, R. A., and Meyerson, A. L., 1986, On the speciation of metals in the water column of a polluted estuary: Marine pollution bulletin, v. 17, no. 12, p. 535-542.
- Ohde, T., and Dadou, I., 2018, Seasonal and annual variability of coastal sulphur plumes in the northern Benguela upwelling system: PloS one, v. 13, no. 2, p. e0192140.
- Petosa, A. R., Jaisi, D. P., Quevedo, I. R., Elimelech, M., and Tufenkji, N., 2010, Aggregation and deposition of engineered nanomaterials in aquatic environments: role of physicochemical interactions: Environmental science & technology, v. 44, no. 17, p. 6532-6549.
- Resing, J. A., Sedwick, P. N., German, C. R., Jenkins, W. J., Moffett, J. W., Sohst, B. M., and Tagliabue, A., 2015, Basin-scale transport of hydrothermal dissolved metals across the South Pacific Ocean: Nature, v. 523, no. 7559, p. 200-203.
- Smith Jr, K. L., Sherman, A. D., Shaw, T. J., and Sprintall, J., 2013, Icebergs as unique Lagrangian ecosystems in polar seas.
- Smith, K. L., Robison, B. H., Helly, J. J., Kaufmann, R. S., Ruhl, H. A., Shaw, T. J., Twining, B. S., and Vernet, M., 2007, Free-drifting icebergs: hot spots of chemical and biological enrichment in the Weddell Sea: science, v. 317, no. 5837, p. 478-482.
- Toner, B. M., Berquó, T. S., Michel, F. M., Sorensen, J. V., Templeton, A. S., and Edwards, K. J., 2012, Mineralogy of iron microbial mats from Loihi Seamount: Frontiers in microbiology, v. 3, p. 118.
- Toner, B. M., German, C. R., Dick, G. J., and Breier, J. A., 2016, Deciphering the Complex Chemistry of Deep-Ocean Particles Using Complementary Synchrotron X-ray Microscope and Microprobe Instruments: Acc Chem Res, v. 49, no. 1, p. 128-137.
- van der Zee, C., Roberts, D. R., Rancourt, D. G., and Slomp, C. P., 2003, Nanogoethite is the dominant reactive oxyhydroxide phase in lake and marine sediments: Geology, v. 31, no. 11, p. 993-996.
- Wells, M. L., and Goldberg, E. D., 1991, Occurrence of small colloids in sea water: Nature, v. 353, no. 6342, p. 342.
- Wells, M. L., and Goldberg, E. D., 1992, Marine submicron particles: Marine Chemistry, v. 40, no. 1-2, p. 5-18.
- Wells, M. L., and Goldberg, E. D., 1994, The distribution of colloids in the North Atlantic and Southern Oceans: Limnology and Oceanography, v. 39, no. 2, p. 286-302.
- Wigginton, N. S., Haus, K. L., and Hochella Jr, M. F., 2007, Aquatic environmental nanoparticles: Journal of Environmental Monitoring, v. 9, no. 12, p. 1306-1316.
Fe Budget, colloids and ocean fertility related to phytoplankton
- Aguilar-Islas, A. M., Wu, J., Rember, R., Johansen, A. M., and Shank, L. M., 2010, Dissolution of aerosol-derived iron in seawater: Leach solution chemistry, aerosol type, and colloidal iron fraction: Marine Chemistry, v. 120, no. 1-4, p. 25-33.
- Bergquist, B. A., Wu, J., and Boyle, E. A., 2007, Variability in oceanic dissolved iron is dominated by the colloidal fraction: Geochimica et Cosmochimica Acta, v. 71, no. 12, p. 2960-2974.
- Blain, S., Queguiner, B., Armand, L., Belviso, S., Bombled, B., Bopp, L., Bowie, A., Brunet, C., Brussaard, C., Carlotti, F., Christaki, U., Corbiere, A., Durand, I., Ebersbach, F., Fuda, J. L., Garcia, N., Gerringa, L., Griffiths, B., Guigue, C., Guillerm, C., Jacquet, S., Jeandel, C., Laan, P., Lefevre, D., Lo Monaco, C., Malits, A., Mosseri, J., Obernosterer, I., Park, Y. H., Picheral, M., Pondaven, P., Remenyi, T., Sandroni, V., Sarthou, G., Savoye, N., Scouarnec, L., Souhaut, M., Thuiller, D., Timmermans, K., Trull, T., Uitz, J., van Beek, P., Veldhuis, M., Vincent, D., Viollier, E., Vong, L., and Wagener, T., 2007, Effect of natural iron fertilization on carbon sequestration in the Southern Ocean: Nature, v. 446, no. 7139, p. 1070-1074.
- Coale, K. H., Johnson, K. S., Fitzwater, S. E., Gordon, R. M., Tanner, S., Chavez, F. P., Ferioli, L., Sakamoto, C., Rogers, P., Millero, F., Steinberg, P., Nightingale, P., Cooper, D., Cochlan, W. P., Landry, M. R., Constantinou, J., Rollwagen, G., Trasvina, A., and Kudela, R., 1996, A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean: Nature, v. 383, p. 495.
- Fitzsimmons, J. N., Boyle, E. A., and Jenkins, W. J., 2014, Distal transport of dissolved hydrothermal iron in the deep South Pacific Ocean: Proceedings of the National Academy of Sciences, v. 111, no. 47, p. 16654-16661.
- Gantt, B., and Meskhidze, N., 2013, The physical and chemical characteristics of marine primary organic aerosol: a review: Atmospheric Chemistry and Physics, v. 13, no. 8, p. 3979-3996.
- Hochella, M. F., Lower, S. K., Maurice, P. A., Penn, R. L., Sahai, N., Sparks, D. L., and Twining, B. S., 2008, Nanominerals, mineral nanoparticles, and earth systems: Science, v. 319, no. 5870, p. 1631-1635.
- Li, W., Xu, L., Liu, X., Zhang, J., Lin, Y., Yao, X., Gao, H., Zhang, D., Chen, J., and Wang, W., 2017, Air pollution–aerosol interactions produce more bioavailable iron for ocean ecosystems: Science advances, v. 3, no. 3, p. e1601749.
- Nishioka, J., Takeda, S., Wong, C. S., and Johnson, W. K., 2001, Size-fractionated iron concentrations in the northeast Pacific Ocean: distribution of soluble and small colloidal iron: Marine Chemistry, v. 74, no. 2-3, p. 157-179.
- Poulton, S. W., and Raiswell, R., 2002, The low-temperature geochemical cycle of iron: from continental fluxes to marine sediment deposition: American Journal of Science, v. 302, no. 9, p. 774-805.
- Raiswell, R., Tranter, M., Benning, L. G., Siegert, M., De'ath, R., Huybrechts, P., and Payne, T., 2006, Contributions from glacially derived sediment to the global iron (oxyhydr)oxide cycle: Implications for iron delivery to the oceans: Geochimica et Cosmochimica Acta, v. 70, no. 11, p. 2765-2780.
- Smith, K. L., Robison, B. H., Helly, J. J., Kaufmann, R. S., Ruhl, H. A., Shaw, T. J., Twining, B. S., and Vernet, M., 2007, Free-drifting icebergs: hot spots of chemical and biological enrichment in the Weddell Sea: Science, v. 317, no. 5837, p. 478-482.
- Wang, S., Bailey, D., Lindsay, K., Moore, J. K., and Holland, M., 2014, Impact of sea ice on the marine iron cycle and phytoplankton productivity: Biogeosciences, v. 11, no. 17, p. 4713-4731.
- Wells, M. L., and Goldberg, E. D., 1991, Occurrence of small colloids in sea water: Nature, v. 353, no. 6342, p. 342.
- Wells, M. L., and Goldberg, E. D., 1994, The distribution of colloids in the North Atlantic and Southern Oceans: Limnology and Oceanography, v. 39, no. 2, p. 286-302.
- Wells, M. L., and Goldberg, E. D., 1992, Marine submicron particles: Marine Chemistry, v. 40, no. 1-2, p. 5-18.
- Wozniak, A. S., Shelley, R. U., McElhenie, S. D., Landing, W. M., and Hatcher, P. G., 2015, Aerosol water soluble organic matter characteristics over the North Atlantic Ocean: Implications for iron-binding ligands and iron solubility: Marine Chemistry, v. 173, p. 162-172.
- Wu, J., Boyle, E., Sunda, W., and Wen, L.-S., 2001, Soluble and colloidal iron in the oligotrophic North Atlantic and North Pacific: Science, v. 293, no. 5531, p. 847-849.
Nanoparticles in Oceans Related to Life/ Biogeochemical Processes
- Canesi, L., and Corsi, I., 2016, Effects of nanomaterials on marine invertebrates: Sci Total Environ, v. 565, p. 933-94
- Eiriksdottir, E. S., Gislason, S. R., and Oelkers, E. H., 2015, Direct evidence of the feedback between climate and nutrient, major, and trace element transport to the oceans: Geochimica et Cosmochimica Acta, v. 166, p. 249-266.
- Luther, G. W., and Rickard, D. T., 2005, Metal Sulfide Cluster Complexes and their Biogeochemical Importance in the Environment: Journal of Nanoparticle Research, v. 7, no. 4-5, p. 389-407.
- Rasmussen, B., Krapež, B., Muhling, J. R., and Suvorova, A., 2015, Precipitation of iron silicate nanoparticles in early Precambrian oceans marks Earth's first iron age: Geology, v. 43, no. 4, p. 303-306.
- Toner, B. M., Berquó, T. S., Michel, F. M., Sorensen, J. V., Templeton, A. S., and Edwards, K. J., 2012, Mineralogy of iron microbial mats from Loihi Seamount: Frontiers in microbiology, v. 3, p. 118.
- Wang, S., Bailey, D., Lindsay, K., Moore, J. K., and Holland, M., 2014, Impact of sea ice on the marine iron cycle and phytoplankton productivity: Biogeosciences, v. 11, no. 17, p. 4713-4731.
Nanoparticles from Submarine Vents--Black Smokers
- Findlay, A. J., Gartman, A., Shaw, T. J., and Luther, G. W., 2015, Trace metal concentration and partitioning in the first 1.5 m of hydrothermal vent plumes along the Mid-Atlantic Ridge: TAG, Snakepit, and Rainbow: Chemical Geology, v. 412, p. 117-131.
- Fitzsimmons, J. N., John, S. G., Marsay, C. M., Hoffman, C. L., Nicholas, S. L., Toner, B. M., German, C. R., and Sherrell, R. M., 2017, Iron persistence in a distal hydrothermal plume supported by dissolved–particulate exchange: Nature Geoscience, v. 10, no. 3, p. 195.
- Gartman, A., and Luther, G. W., 2013, Comparison of pyrite (FeS2) synthesis mechanisms to reproduce natural FeS2 nanoparticles found at hydrothermal vents: Geochimica et Cosmochimica Acta, v. 120, p. 447-458.
- Gartman, A., and Luther, G. W., 2014, Oxidation of synthesized sub-micron pyrite (FeS2) in seawater: Geochimica et Cosmochimica Acta, v. 144, p. 96-108.
- Gartman, A., Findlay, A. J., and Luther, G. W., 2014, Nanoparticulate pyrite and other nanoparticles are a widespread component of hydrothermal vent black smoker emissions: Chemical Geology, v. 366, p. 32-41.
- Gartman, A., Hannington, M., Jamieson, J. W., Peterkin, B., Garbe-Schönberg, D., Findlay, A. J., Fuchs, S., and Kwasnitschka, T., 2017, Boiling-induced formation of colloidal gold in black smoker hydrothermal fluids: Geology, v. 46, no. 1, p. 39-42.
- Li, M., Toner, B. M., Baker, B. J., Breier, J. A., Sheik, C. S., and Dick, G. J., 2014, Microbial iron uptake as a mechanism for dispersing iron from deep-sea hydrothermal vents: Nature communications, v. 5, p. 3192.
- Yucel, M., Gartman, A., Chan, C. S., and Luther, G. W., 2011, Hydrothermal vents as a kinetically stable source of iron-sulphide-bearing nanoparticles to the ocean. Nat. Geosci. 4, 367
Nanoparticles in Fresh Water
- Alimi, O. S., Farner Budarz, J., Hernandez, L. M., and Tufenkji, N., 2018, Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport: Environ Sci Technol, v. 52, no. 4, p. 1704-1724.
- Batley, G. E., Kirby, J. K., and McLaughlin, M. J., 2013, Fate and Risks of Nanomaterials in Aquatic and Terrestrial Environments: Accounts of Chemical Research, v. 46, no. 3, p. 854-862.
- Civeira, M. S., Ramos, C. G., Oliveira, M. L., Kautzmann, R. M., Taffarel, S. R., Teixeira, E. C., and Silva, L. F., 2016, Nano-mineralogy of suspended sediment during the beginning of coal rejects spill: Chemosphere, v. 145, p. 142-147.
- Geitner, N. K., Cooper, J. L., Avellan, A., Castellon, B. T., Perrotta, B. G., Bossa, N., Simonin, M., Anderson, S. M., Inoue, S., Hochella, M. F., Jr., Richardson, C. J., Bernhardt, E. S., Lowry, G. V., Ferguson, P. L., Matson, C. W., King, R. S., Unrine, J. M., Wiesner, M. R., and Hsu-Kim, H., 2018, Size-Based Differential Transport, Uptake, and Mass Distribution of Ceria (CeO2) Nanoparticles in Wetland Mesocosms: Environ Sci Technol, v. 52, no. 17, p. 9768-9776.
- Kim, B., Richardson, C. J., Murayama, M., and Hochella, M. F., 2013, Nanoscale Analytical Transmission Electron Microscopy Techniques Applicable to Wetland Research and Monitoring: Methods in Biogeochemistry of Wetlands, no. methodsinbiogeo, p. 857-878
- Kyle, J. E., Eydal, H. S., Ferris, F. G., and Pedersen, K., 2008, Viruses in granitic groundwater from 69 to 450 m depth of the Aspo hard rock laboratory, Sweden: ISME J, v. 2, no. 5, p. 571-574.
- Lapworth, D. J., Stolpe, B., Williams, P. J., Gooddy, D. C., and Lead, J. R., 2013, Characterization of suboxic groundwater colloids using a multi-method approach: Environ Sci Technol, v. 47, no. 6, p. 2554-2561.
- Lee, S., Shen, Z., and Xu, H., 2016, Study on nanophase iron oxyhydroxides in freshwater ferromanganese nodules from Green Bay, Lake Michigan, with implications for the adsorption of As and heavy metals: American Mineralogist, v. 101, no. 9, p. 1986-1995.
- Mattsson, K., Hansson, L. A., and Cedervall, T., 2015, Nano-plastics in the aquatic environment: Environmental Science: Processes & Impacts, v. 17, no. 10, p. 1712-1721.
- Mitrano, D. M., Mehrabi, K., Dasilva, Y. A. R., and Nowack, B., 2017, Mobility of metallic (nano)particles in leachates from landfills containing waste incineration residues: Environmental Science: Nano, v. 4, no. 2, p. 480-492.
- Petosa, A. R., Jaisi, D. P., Quevedo, I. R., Elimelech, M., and Tufenkji, N., 2010, Aggregation and deposition of engineered nanomaterials in aquatic environments: role of physicochemical interactions: Environmental science & technology, v. 44, no. 17, p. 6532-6549.
- Rozan, T. F., Luther, G. W., Ridge, D., and Robinson, S., 2003, Determination of Pb complexation in oxic and sulfidic waters using pseudovoltammetry: Environmental science & technology, v. 37, no. 17, p. 3845-3852.
- Simonin, M., Colman, B. P., Anderson, S. M., King, R. S., Ruis, M. T., Avellan, A., Bergemann, C. M., Perrotta, B. G., Geitner, N. K., and Ho, M., 2018, Engineered nanoparticles interact with nutrients to intensify eutrophication in a wetland ecosystem experiment: Ecological Applications, v. 28, no. 6, p. 1435-1449.
- Smith Jr, K. L., Sherman, A. D., Shaw, T. J., and Sprintall, J., 2013, Icebergs as unique Lagrangian ecosystems in polar seas.
- Smith, K. L., Robison, B. H., Helly, J. J., Kaufmann, R. S., Ruhl, H. A., Shaw, T. J., Twining, B. S., and Vernet, M., 2007, Free-drifting icebergs: hot spots of chemical and biological enrichment in the Weddell Sea: science, v. 317, no. 5837, p. 478-482.
- van der Zee, C., Roberts, D. R., Rancourt, D. G., and Slomp, C. P., 2003, Nanogoethite is the dominant reactive oxyhydroxide phase in lake and marine sediments: Geology, v. 31, no. 11, p. 993-996.
- Von der Heyden, B., Roychoudhury, A., and Myneni, S., 2019, Iron-Rich Nanoparticles in Natural Aquatic Environments, Minerals, v. 9, 287 p. doi:10.3390/min9050287
- Yang, Y., Colman, B. P., Bernhardt, E. S., and Hochella, M. F., 2015, Importance of a nanoscience approach in the understanding of major aqueous contamination scenarios: case study from a recent coal ash spill: Environmental science & technology, v. 49, no. 6, p. 3375-3382.
Nanoparticles in Rivers
- Espana, J. S., Pamo, E. L., Santofimia, E., Aduvire, O., Reyes, J., and Barettino, D., 2005, Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): geochemistry, mineralogy and environmental implications: Applied geochemistry, v. 20, no. 7, p. 1320-1356.
- Olías, M., Cánovas, C. R., Nieto, J. M., and Sarmiento, A. M., 2006, Evaluation of the dissolved contaminant load transported by the Tinto and Odiel rivers (South West Spain): Applied Geochemistry, v. 21, no. 10, p. 1733-1749.
- Poulton, S. W., and Raiswell, R., 2005, Chemical and physical characteristics of iron oxides in riverine and glacial meltwater sediments: Chemical Geology, v. 218, no. 3-4, p. 203-221
- Rozan, T. F., Lassman, M. E., Ridge, D. P., and Luther III, G. W., 2000, Evidence for iron, copper and zinc complexation as multinuclear sulphide clusters in oxic rivers: Nature, v. 406, no. 6798, p. 879.
- Rozan, T. F., and Luther III, G. W., 2002, Voltammetric evidence suggesting Ag speciation is dominated by sulfide complexation in river water, ACS Publications.
Nanoparticles and Waste Water Treatment
- Grant, S. B., Saphores, J.-D., Feldman, D. L., Hamilton, A. J., Fletcher, T. D., Cook, P. L., Stewardson, M., Sanders, B. F., Levin, L. A., and Ambrose, R. F., 2012, Taking the "waste" out of "wastewater" for human water security and ecosystem sustainability: science, v. 337, no. 6095, p. 681-686.
- Kim, B., Park, C.-S., Murayama, M., and Hochella Jr, M. F., 2010, Discovery and characterization of silver sulfide nanoparticles in final sewage sludge products: Environmental science & technology, v. 44, no. 19, p. 7509-7514.
- Kim, B., Murayama, M., Colman, B. P., and Hochella, M. F., 2012, Characterization and environmental implications of nano-and larger TiO 2 particles in sewage sludge, and soils amended with sewage sludge: Journal of Environmental Monitoring, v. 14, no. 4, p. 1128-1136.
- Kim, B., Richardson, C. J., Murayama, M., and Hochella, M. F., 2013, Nanoscale Analytical Transmission Electron Microscopy Techniques Applicable to Wetland Research and Monitoring: Methods in Biogeochemistry of Wetlands, no. methodsinbiogeo, p. 857-878.
- Kiser, M. A., Ryu, H., Jang, H., Hristovski, K., and Westerhoff, P., 2010, Biosorption of nanoparticles to heterotrophic wastewater biomass: Water Research, v. 44, no. 14, p. 4105-4114.
- Lowry, G. V., Espinasse, B. P., Badireddy, A. R., Richardson, C. J., Reinsch, B. C., Bryant, L. D., Bone, A. J., Deonarine, A., Chae, S., Therezien, M., Colman, B. P., Hsu-Kim, H., Bernhardt, E. S., Matson, C. W., and Wiesner, M. R., 2012, Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large scale freshwater emergent wetland: Environ Sci Technol, v. 46, no. 13, p. 7027-7036.
- Tou, F., Yang, Y., Feng, J., Niu, Z., Pan, H., Qin, Y., Guo, X., Meng, X., Liu, M., and Hochella, M. F., 2017, Environmental Risk Implications of Metals in Sludges from Waste Water Treatment Plants: The Discovery of Vast Stores of Metal-Containing Nanoparticles: Environmental Science & Technology, v. 51, no. 9, p. 4831-4840.
- Vriens, B., Voegelin, A., Hug, S. J., Kaegi, R., Winkel, L. H., Buser, A. M., and Berg, M., 2017, Quantification of Element Fluxes in Wastewaters: A Nationwide Survey in Switzerland: Environmental science & technology, v. 51, no. 19, p. 10943-10953.
- Westerhoff, P., Lee, S., Yang, Y., Gordon, G. W., Hristovski, K., Halden, R. U., and Herckes, P., 2015, Characterization, recovery opportunities, and valuation of metals in municipal sludges from US wastewater treatment plants nationwide: Environmental science & technology, v. 49, no. 16, p. 9479-9488.
- Westerhoff, P., Song, G., Hristovski, K., and Kiser, M. A., 2011, Occurrence and removal of titanium at full scale wastewater treatment plants: implications for TiO 2 nanomaterials: Journal of Environmental Monitoring, v. 13, no. 5, p. 1195-1203.
- Westerhoff, P. K., Kiser, M. A., and Hristovski, K., 2013, Nanomaterial removal and transformation during biological wastewater treatment: Environmental engineering science, v. 30, no. 3, p. 109-117.
- Westerhoff, P., Alvarez, P., Li, Q., Gardea-Torresdey, J., and Zimmerman, J., 2016, Overcoming implementation barriers for nanotechnology in drinking water treatment: Environmental Science: Nano, v. 3, no. 6, p. 1241-1253.
Nanoparticles In the Atmosphere/Aerosols/Airborne Pollutants

Scale of Nanoparticles in the Atmosphere
![[creative commons]](/images/creativecommons_16.png)
Provenance: Image from Deutscher Wetterdienst, Particle Size Distribution https://www.dwd.de/EN/research/observing_atmosphere/composition_atmosphere/aerosol/cont_nav/particle_size_distribution_node.html
Reuse: This item is offered under a Creative Commons Attribution-NonCommercial-ShareAlike license http://creativecommons.org/licenses/by-nc-sa/3.0/ You may reuse this item for non-commercial purposes as long as you provide attribution and offer any derivative works under a similar license.
Processes and Pathways
- Visit the comprehensive website Deutscher Wetterdienst (Germany but text is in English). Extensive information on methods of detecting particles in the atmosphere: Atmospheric composition, trace gases, ozone profile, aerosols, total suspended particles, particle number-concentration, particle size distribution, scattering and absoroption, aerosol optical depth....
- Clarke, A. D., Owens, S. R., and Zhou, J., 2006, An ultrafine sea‐salt flux from breaking waves: Implications for cloud condensation nuclei in the remote marine atmosphere: Journal of Geophysical Research: Atmospheres, v. 111, no. D6.
- Coggon, M. M., Sorooshian, A., Wang, Z., Metcalf, A. R., Frossard, A. A., Lin, J. J., Craven, J. S., Nenes, A., Jonsson, H. H., and Russell, L. M., 2012, Ship impacts on the marine atmosphere: insights into the contribution of shipping emissions to the properties of marine aerosol and clouds: Atmospheric Chemistry and Physics, v. 12, no. 18, p. 8439-8458.
- Durdina, L., Brem, B. T., Setyan, A., Siegerist, F., Rindlisbacher, T., and Wang, J., 2017, Assessment of particle pollution from jetliners: from smoke visibility to nanoparticle counting: Environmental science & technology, v. 51, no. 6, p. 3534-3541.
- Huang, Y., Shen, H., Chen, H., Wang, R., Zhang, Y., Su, S., Chen, Y., Lin, N., Zhuo, S., Zhong, Q., Wang, X., Liu, J., Li, B., Liu, W., and Tao, S., 2014, Quantification of global primary emissions of PM2.5, PM10, and TSP from combustion and industrial process sources: Environ Sci Technol, v. 48, no. 23, p. 13834-13843.
- Kulmala, M., 2003, How particles nucleate and grow: Science, v. 302, no. 5647, p. 1000-1001.
- Kulmala, M., Petaja, T., Ehn, M., Thornton, J., Sipila, M., Worsnop, D. R., and Kerminen, V. M., 2014, Chemistry of Atmospheric Nucleation: On the Recent Advances on Precursor Characterization and Atmospheric Cluster Composition in Connection with Atmospheric New Particle Formation, in Johnson, M. A., and Martinez, T. J., eds., Annual Review of Physical Chemistry, Vol 65, Volume 65: Palo Alto, Annual Reviews, p. 21-37.
- Kumar, P., and Al-Dabbous, A. N., 2016, Emission, transformation and fate of nanoparticles in the atmosphere: in Engineered Nanoparticles and the Environment: Biophysicochemical Processes and Biotoxicity, N. Senesi, Ed, v. 4.
- Li, W., Xu, L., Liu, X., Zhang, J., Lin, Y., Yao, X., Gao, H., Zhang, D., Chen, J., and Wang, W., 2017, Air pollution–aerosol interactions produce more bioavailable iron for ocean ecosystems: Science advances, v. 3, no. 3, p. e1601749.
- Lohmann, U., and Feichter, J., 2005, Global indirect aerosol effects: a review: Atmospheric Chemistry and Physics, v. 5, no. 3, p. 715-737.
- Mackinnon, I. D. R., and Mogk, D. W., 1985, Surface sulfur measurements on stratospheric particles: Geophysical research letters, v. 12, no. 2, p. 93-96.
- May, A. A., Nguyen, N. T., Presto, A. A., Gordon, T. D., Lipsky, E. M., Karve, M., Gutierrez, A., Robertson, W. H., Zhang, M., and Brandow, C., 2014, Gas-and particle-phase primary emissions from in-use, on-road gasoline and diesel vehicles: Atmospheric Environment, v. 88, p. 247-260.
- Mead, C., Herckes, P., Majestic, B. J., and Anbar, A. D., 2013, Source apportionment of aerosol iron in the marine environment using iron isotope analysis: Geophysical Research Letters, v. 40, no. 21, p. 5722-5727.
- Merikanto, J., Spracklen, D. V., Mann, G. W., Pickering, S. J., and Carslaw, K. S., 2009, Impact of nucleation on global CCN: Atmospheric Chemistry and Physics, v. 9, no. 21, p. 8601-8616.
- Mohnen, V., and Hidy, G. M., 2010, Measurements of atmospheric nanoparticles (1875–1980): Bulletin of the American Meteorological Society, v. 91, no. 11, p. 1525-1540.
- Murr, L. E., and Bang, J. J., 2003, Electron microscope comparisons of fine and ultra-fine carbonaceous and non-carbonaceous, airborne particulates: Atmospheric Environment, v. 37, no. 34, p. 4795-4806.
- O'Dowd, C. D., Smith, M. H., Consterdine, I. E., and Lowe, J. A., 1997, Marine aerosol, sea-salt, and the marine sulphur cycle: A short review: Atmospheric Environment, v. 31, no. 1, p. 73-80.
- O'Dowd, D. C., and De Leeuw, G., 2007, Marine aerosol production: a review of the current knowledge: Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences, v. 365, no. 1856, p. 1753-1774.
- Peters, T. M., Elzey, S., Johnson, R., Park, H., Grassian, V. H., Maher, T., and O'Shaughnessy, P., 2009, Airborne monitoring to distinguish engineered nanomaterials from incidental particles for environmental health and safety: J Occup Environ Hyg, v. 6, no. 2, p. 73-81.
- Posner, L. N., and Pandis, S. N., 2015, Sources of ultrafine particles in the Eastern United States: Atmospheric Environment, v. 111, p. 103-112.
- Prather, K. A., Bertram, T. H., Grassian, V. H., Deane, G. B., Stokes, M. D., DeMott, P. J., Aluwihare, L. I., Palenik, B. P., Azam, F., and Seinfeld, J. H., 2013, Bringing the ocean into the laboratory to probe the chemical complexity of sea spray aerosol: Proceedings of the National Academy of Sciences, v. 110, no. 19, p. 7550-7555.
- Ramanathan, V., Crutzen, P., Kiehl, J., and Rosenfeld, D., 2001, Aerosols, climate, and the hydrological cycle: Science, v. 294, no. 5549, p. 2119-2124.
- Vehkamäki, H., and Riipinen, I., 2012, Thermodynamics and kinetics of atmospheric aerosol particle formation and growth: Chemical Society Reviews, v. 41, no. 15, p. 5160-5173. "In this tutorial review we summarize the standard approaches to describe aerosol formation from atmospheric vapours and subsequent growth–with a particular emphasis on the interplay between equilibrium thermodynamics and non-equilibrium transport"
- Wozniak, A. S., Shelley, R. U., McElhenie, S. D., Landing, W. M., and Hatcher, P. G., 2015, Aerosol water soluble organic matter characteristics over the North Atlantic Ocean: Implications for iron-binding ligands and iron solubility: Marine Chemistry, v. 173, p. 162-172.
- Yang, Y., Vance, M., Tou, F., Tiwari, A., Liu, M., and Hochella, M. F., 2016, Nanoparticles in road dust from impervious urban surfaces: distribution, identification, and environmental implications: Environmental Science: Nano, v. 3, no. 3, p. 534-544.
- Zetterdahl, M., Moldanová, J., Pei, X., Pathak, R. K., and Demirdjian, B., 2016, Impact of the 0.1% fuel sulfur content limit in SECA on particle and gaseous emissions from marine vessels: Atmospheric Environment, v. 145, p. 338-345.
- Zheng, M., Salmon, L. G., Schauer, J. J., Zeng, L., Kiang, C. S., Zhang, Y., and Cass, G. R., 2005, Seasonal trends in PM2. 5 source contributions in Beijing, China: Atmospheric Environment, v. 39, no. 22, p. 3967-3976.

Airborne particles are commonly either biological contaminants, particulate contaminants, gaseous contaminants, or dust. This diagram shows the size distribution in micrometres (µm) of various types of airborne particles.
![[reuse info]](/images/information_16.png)
Provenance: Source: Wikipedia; https://en.wikipedia.org/wiki/File:Airborne-particulate-size-chart.svg
Reuse: Permission is granted to copy, distribute and/or modify this document under the terms of the GNU Free Documentation License, Version 1.2 or any later version published by the Free Software Foundation; with no Invariant Sections, no Front-Cover Texts, and no Back-Cover Texts.
Natural Particles
- Buseck, P. R., and Posfai, M., 1999, Airborne minerals and related aerosol particles: Effects on climate and the environment: Proceedings of the National Academy of Sciences, v. 96, no. 7, p. 3372-3379.
- Buseck, P. R., and Adachi, K., 2008, Nanoparticles in the atmosphere: Elements, v. 4, no. 6, p. 389-394.
- Gantt, B., and Meskhidze, N., 2013, The physical and chemical characteristics of marine primary organic aerosol: a review: Atmospheric Chemistry and Physics, v. 13, no. 8, p. 3979-3996.
- Morton Peter L., M., L. W., Shih‐Chieh, H., Angela, M., Yuan, G., Susan, G., G., H. M., Mariko, H., M., J. A., Rémi, L., Chris, M., D., P. M., Gretchen, S., Amanda, V., and M., Z. L., 2013, Methods for the sampling and analysis of marine aerosols: results from the 2008 GEOTRACES aerosol intercalibration experiment: Limnology and Oceanography: Methods, v. 11, no. 2, p. 62-78.
- Tegen, I., Werner, M., Harrison, S. P., and Kohfeld, K. E., 2004, Relative importance of climate and land use in determining present and future global soil dust emission: Geophysical Research Letters, v. 31, no. 5.
Anthropogenic Particles
- Johnson, D. R., 2016, Nanometer-sized emissions from municipal waste incinerators: A qualitative risk assessment: J Hazard Mater, v. 320, p. 67-79.
- Kumar, P., Robins, A., Vardoulakis, S., and Britter, R., 2010, A review of the characteristics of nanoparticles in the urban atmosphere and the prospects for developing regulatory controls: Atmospheric Environment, v. 44, no. 39, p. 5035-5052.
- Kumar, P., Robins, A., Vardoulakis, S., and Britter, R., 2010, A review of the characteristics of nanoparticles in the urban atmosphere and the prospects for developing regulatory controls: Atmospheric Environment, v. 44, no. 39, p. 5035-5052.
- Kumar, P., and Al-Dabbous, A. N., 2016, Emission, transformation and fate of nanoparticles in the atmosphere: in Engineered Nanoparticles and the Environment: Biophysicochemical Processes and Biotoxicity, N. Senesi, Ed, v. 4.
- Mackinnon, I. D. R., and Mogk, D. W., 1985, Surface sulfur measurements on stratospheric particles: Geophysical research letters, v. 12, no. 2, p. 93-9
- Niu, J., Rasmussen, P. E., Magee, R., and Nilsson, G., 2015, Spatial and temporal variability of incidental nanoparticles in indoor workplaces: impact on the characterization of point source exposures: Environ Sci Process Impacts, v. 17, no. 1, p. 98-109.
- Peters, T. M., Elzey, S., Johnson, R., Park, H., Grassian, V. H., Maher, T., and O'Shaughnessy, P., 2009, Airborne monitoring to distinguish engineered nanomaterials from incidental particles for environmental health and safety: J Occup Environ Hyg, v. 6, no. 2, p. 73-81.
- Posner, L. N., and Pandis, S. N., 2015, Sources of ultrafine particles in the Eastern United States: Atmospheric Environment, v. 111, p. 103-112
- Seigneur, C., 2009, Current understanding of ultrafine particulate matter emitted from mobile sources: Journal of the Air & Waste Management Association, v. 59, no. 1, p. 3-17.
- Utsunomiya, S., Jensen, K. A., Keeler, G. J., and Ewing, R. C., 2004, Direct identification of trace metals in fine and ultrafine particles in the Detroit urban atmosphere: Environmental Science & Technology, v. 38, no. 8, p. 2289-2297.
- Yang, Y., Vance, M., Tou, F., Tiwari, A., Liu, M., and Hochella, M. F., 2016, Nanoparticles in road dust from impervious urban surfaces: distribution, identification, and environmental implications: Environmental Science: Nano, v. 3, no. 3, p. 534-544.
- Zheng, M., Salmon, L. G., Schauer, J. J., Zeng, L., Kiang, C. S., Zhang, Y., and Cass, G. R., 2005, Seasonal trends in PM2. 5 source contributions in Beijing, China: Atmospheric Environment, v. 39, no. 22, p. 3967-3976.
Nanoparticles and Climate Change

Sources and appearance of atmospheric aerosols.
Top: local and large scale air pollution. Sources include (bottom, counterclockwise) volcanic eruptions (producing volcanic ash and sulphate), sea spray (sea salt and sulphate aerosols), desert storms (mineral dust), savannah biomass burning (BC and OC), coal power plants (fossil fuel BC and OC, sulphate, nitrate), ships (BC, OC, sulphates, nitrate), cooking* (domestic BC and OC), road transport (sulphate, BC, VOCs yielding OC). Center: Electron microscope images of (A) sulphates, (B) soot, (C) fly ash, a product of coal combustion (Posfai et al., 1999).
© 2013 Nature Education Images courtesy of Eyjafjallajökull eruption: courtesy of Árni Friðriksson, Wikimedia commons; Sea spray: NASA/JPL; Desert storm: Wikimedia commons; Savannah biomass burning: Wikimedia Commons ; Coal power plants: Wikimedia Commons; Ship in a Norwegian fjord: Stefan Großmann, Wikimedia commons; Cooking: Fullerton et al.2009; Truck: U. S. EPA, Wikimedia commons. All rights reserved.
![[creative commons]](/images/creativecommons_16.png)
Provenance: Image From Nature Education Scitable; https://www.nature.com/scitable/knowledge/library/aerosols-and-their-relation-to-global-climate-102215345 Terms of use of this image: You may reproduce this material, without modifications, in print or electronic form for your personal, non-commercial purposes or for non-commercial use in an educational environment.
Reuse: This item is offered under a Creative Commons Attribution-NonCommercial-ShareAlike license http://creativecommons.org/licenses/by-nc-sa/3.0/ You may reuse this item for non-commercial purposes as long as you provide attribution and offer any derivative works under a similar license.
- Booth, B. B., Dunstone, N. J., Halloran, P. R., Andrews, T., and Bellouin, N., 2012, Aerosols implicated as a prime driver of twentieth-century North Atlantic climate variability: Nature, v. 484, no. 7393, p. 228-232.
- Gislason, S. R., Oelkers, E. H., Eiriksdottir, E. S., Kardjilov, M. I., Gisladottir, G., Sigfusson, B., Snorrason, A., Elefsen, S., Hardardottir, J., Torssander, P., and Oskarsson, N., 2009, Direct evidence of the feedback between climate and weathering: Earth and Planetary Science Letters, v. 277, no. 1-2, p. 213-222.
- Hällström, N., 2008, What Next? Climate change, technology and development: Development, v. 51, no. 3, p. 375-381.
- Mahowald, N., 2011, Aerosol indirect effect on biogeochemical cycles and climate: Science, v. 334, no. 6057, p. 794-796.
- Mahowald, N., Ward, D. S., Kloster, S., Flanner, M. G., Heald, C. L., Heavens, N. G., Hess, P. G., Lamarque, J.-F., and Chuang, P. Y., 2011, Aerosol Impacts on Climate and Biogeochemistry: Annual Review of Environment and Resources, v. 36, no. 1, p. 45-74.
- Myhre, G., Myhre, C. L., Samset, B., and Storelvmo, T., 2013, Aerosols and their relation to global climate and climate sensitivity: Nature Education Knowledge, v. 4, no. 5, p. 7.
- Prospero, J. M., and Lamb, P. J., 2003, African droughts and dust transport to the Caribbean: Climate change implications: Science, v. 302, no. 5647, p. 1024-1027.
- Qafoku, N. P., 2015, Climate-change effects on soils: accelerated weathering, soil carbon, and elemental cycling, Advances in Agronomy, Volume 131, Elsevier, p. 111-172.
- Ramanathan, V., Crutzen, P., Kiehl, J., and Rosenfeld, D., 2001, Aerosols, climate, and the hydrological cycle: Science, v. 294, no. 5549, p. 2119-2124.
- Tegen, I., Werner, M., Harrison, S. P., and Kohfeld, K. E., 2004, Relative importance of climate and land use in determining present and future global soil dust emission: Geophysical Research Letters, v. 31, no. 5.
Nanoparticles and Carbon Sequestration
- Austrheim, H., Putnis, C. V., and Ruiz-Agudo, E., 2012, Direct Nanoscale Observations of CO2 Sequestration during Brucite [Mg (OH)2] Dissolution: Environmental Science & Technology.
- Blain, S., Queguiner, B., Armand, L., Belviso, S., Bombled, B., Bopp, L., Bowie, A., Brunet, C., Brussaard, C., Carlotti, F., Christaki, U., Corbiere, A., Durand, I., Ebersbach, F., Fuda, J. L., Garcia, N., Gerringa, L., Griffiths, B., Guigue, C., Guillerm, C., Jacquet, S., Jeandel, C., Laan, P., Lefevre, D., Lo Monaco, C., Malits, A., Mosseri, J., Obernosterer, I., Park, Y. H., Picheral, M., Pondaven, P., Remenyi, T., Sandroni, V., Sarthou, G., Savoye, N., Scouarnec, L., Souhaut, M., Thuiller, D., Timmermans, K., Trull, T., Uitz, J., van Beek, P., Veldhuis, M., Vincent, D., Viollier, E., Vong, L., and Wagener, T., 2007, Effect of natural iron fertilization on carbon sequestration in the Southern Ocean: Nature, v. 446, no. 7139, p. 1070-1074.
- Hamilton, J. L., Wilson, S. A., Morgan, B., Turvey, C. C., Paterson, D. J., MacRae, C., McCutcheon, J., and Southam, G., 2016, Nesquehonite sequesters transition metals and CO2 during accelerated carbon mineralisation: International Journal of Greenhouse Gas Control, v. 55, p. 73-81
- Hövelmann, J., Putnis, C. V., Ruiz-Agudo, E., and Austrheim, H., 2012, Direct Nanoscale Observations of CO2 Sequestration during Brucite [Mg(OH)2] Dissolution: Environmental Science & Technology, v. 46, no. 9, p. 5253-5260.
- Lawter, A. R., Qafoku, N. P., Asmussen, R. M., Kukkadapu, R. K., Qafoku, O., Bacon, D. H., and Brown, C. F., 2018, Element mobilization and immobilization from carbonate rocks between CO2 storage reservoirs and the overlying aquifers during a potential CO2 leakage: Chemosphereage reservoirs and the overlying aquifers during a potential CO2 leakage: Chemosphere
- Liu, J., Thallapally, P. K., McGrail, B. P., Brown, D. R., and Liu, J., 2012, Progress in adsorption-based CO2 capture by metal-organic frameworks: Chem Soc Rev, v. 41, no. 6, p. 2308-2322.
- McGrail, B. P., Schaef, H. T., Ho, A. M., Chien, Y.-J., Dooley, J. J., and Davidson, C. L., 2006, Potential for carbon dioxide sequestration in flood basalts: Journal of Geophysical Research: Solid Earth, v. 111, no. B12, p. n/a-n/a.
Nanoparticles and Coal/Biomass Combustion
- Chakrabarty, R. K., Moosmuller, H., Chen, L. W. A., Lewis, K., Arnott, W. P., Mazzoleni, C., Dubey, M. K., Wold, C. E., Hao, W. M., and Kreidenweis, S. M., 2010, Brown carbon in tar balls from smoldering biomass combustion: Atmospheric Chemistry and Physics, v. 10, no. 13, p. 6363-6370.
- Engle, M. A., Radke, L. F., Heffern, E. L., O'Keefe, J. M., Hower, J. C., Smeltzer, C. D., Hower, J. M., Olea, R. A., Eatwell, R. J., Blake, D. R., Emsbo-Mattingly, S. D., Stout, S. A., Queen, G., Aggen, K. L., Kolker, A., Prakash, A., Henke, K. R., Stracher, G. B., Schroeder, P. A., Roman-Colon, Y., and ter Schure, A., 2012, Gas emissions, minerals, and tars associated with three coal fires, Powder River Basin, USA: Sci Total Environ, v. 420, p. 146-159.
- Ma, Q., Cai, S., Wang, S., Zhao, B., Martin, R. V., Brauer, M., Cohen, A., Jiang, J., Zhou, W., and Hao, J., 2017, Impacts of coal burning on ambient PM 2.5 pollution in China: Atmospheric Chemistry and Physics, v. 17, no. 7, p. 4477-4491.
- Martinello, K., Oliveira, M. L. S., Molossi, F. A., Ramos, C. G., Teixeira, E. C., Kautzmann, R. M., and Silva, L. F. O., 2014, Direct identification of hazardous elements in ultra-fine and nanominerals from coal fly ash produced during diesel co-firing: Science of the Total Environment, v. 470, p. 444-452.
- Pone, J. D. N., Hein, K. A. A., Stracher, G. B., Annegarn, H. J., Finkleman, R. B., Blake, D. R., McCormack, J. K., and Schroeder, P., 2007, The spontaneous combustion of coal and its by-products in the Witbank and Sasolburg coalfields of South Africa: International Journal of Coal Geology, v. 72, no. 2, p. 124-140.
- Scheffknecht,G., L. Al-Makhadmeh, U. Schnell, J. Maier, Oxy-fuel coal combustion—A review of the current state-of-the-art. International Journal of Greenhouse Gas Control 5, S16-S35 (2011).
- Stracher, G. B., Prakash, A., Schroeder, P., McCormack, J., Zhang, X., Van Dijk, P., and Blake, D., 2005, New mineral occurrences and mineralization processes: Wuda coal-fire gas vents of Inner Mongolia: American Mineralogist, v. 90, no. 11-12, p. 1729-1739.
- Yang, Y., Colman, B. P., Bernhardt, E. S., and Hochella, M. F., 2015, Importance of a nanoscience approach in the understanding of major aqueous contamination scenarios: case study from a recent coal ash spill: Environmental science & technology, v. 49, no. 6, p. 3375-3382.
- Yi, H., Guo, X., Hao, J., Duan, L., and Li, X., 2006, Characteristics of inhalable particulate matter concentration and size distribution from power plants in China: Journal of the Air & Waste Management Association, v. 56, no. 9, p. 1243-1251.
Discovery and ramifications of incidental Magnéli phase generation and release from industrial coal burning
- Yang, Y., Chen, B., Hower, J., Schindler, M., Winkler, C., Brandt, J., Giulio, R., Ge, J., Liu, M., and Fu, Y., 2017, Discovery and ramifications of incidental Magnéli phase generation and release from industrial coal-burning: Nature communications, v. 8, no. 1, p. 194. Hochella reports: "The Magnéli phases of Ti oxides comprise a series of nonstoichiometric compounds with a generic formula TinO2n−1, where n is a number ranges from 4 to 10. These materials caused great attention due to their significant electrical conductivity and photocatalytic activity under visible light. All these phases possess a triclinic system and very similar lattice parameters. The Magnéli phases are built up of TiO6 octahedra which share corners and edges to form slabs of rutile. Usually, the Magnéli phases are obtained at high temperature in reductive atmosphere, including reducing TiO2 by carbon or ammonia.
Nanoparticles and Geoengineering
- Rasch, P. J., Tilmes, S., Turco, R. P., Robock, A., Oman, L., Chen, C.-C. J., Stenchikov, G. L., and Garcia, R. R., 2008, An overview of geoengineering of climate using stratospheric sulphate aerosols: Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences, v. 366, no. 1882, p. 4007-4037.
- Temple, J., 2018, Will the world ever be ready for solar geoengineering?(vol 96, pg 28, 2018): Chemical & Engineering News, v. 96, no. 14, p. 5-5.
Nanoparticles in Soils and the Critical Zone
- See the module on Teaching Clay Mineralogy from the Teaching Mineralogy module from the On the Cutting Edge program for Geoscience Faculty Professional Development.
- Benny K. G. Theng, G. Y., 2008, Nanoparticles in the Soil Environment: Elements, v. 4, no. 6, p. 395-399.
- Burkhardt, E.-M., Akob, D. M., Bischoff, S., Sitte, J., Kostka, J. E., Banerjee, D., Scheinost, A. C., and Küsel, K., 2009, Impact of biostimulated redox processes on metal dynamics in an iron-rich creek soil of a former uranium mining area: Environmental science & technology, v. 44, no. 1, p. 177-183.
- Dearing, J. A., Hay, K. L., Baban, S. M. J., Huddleston, A. S., Wellington, E. M. H., and Loveland, P. J., 1996, Magnetic susceptibility of soil: An evaluation of conflicting theories using a national data set: Geophysical Journal International, v. 127, no. 3, p. 728-734.
- Hufschmid, R., Newcomb, C. J., Grate, J. W., De Yoreo, J. J., Browning, N. D., and Qafoku, N. P., 2017, Direct Visualization of Aggregate Morphology and Dynamics in a Model Soil Organic–Mineral System: Environmental Science & Technology Letters, v. 4, no. 5, p. 186-191.
- Johnston, C. T., 2010, Probing the nanoscale architecture of clay minerals: Clay Minerals, v. 45, no. 3, p. 245-279.
- Kabengi, N. J., & Thompson, A. , 2011, The emerging emphasis on nanometer-scale processes in soil environments: Soil Science Society of America Journal, v. 75, no. 2, p. 333-334.
- Lusby, G., Hall, C., and Reiners, J., 2015, Lead Contamination of Surface Soils in Philadelphia from Lead Smelters and Urbanization: Environmental Justice, v. 8, no. 1, p. 6-14.
- Maurice, P. A., and Hochella, M. F., 2008, Chapter 5 Nanoscale Particles and Processes: A New Dimension in Soil Science, Advances in Agronomy, Volume 100, Academic Press, p. 123-153.
- Maurice, P. A., 2010, Soil Science at the Nanoscale: A New View of Structure, Stability, and Reactivity, Molecular Environmental Soil Science at the Interfaces in the Earth's Critical Zone, Springer, p. 243-245.
- Mueller, C. W., Weber, P. K., Kilburn, M. R., Hoeschen, C., Kleber, M., and Pett-Ridge, J., 2013, Advances in the analysis of biogeochemical interfaces: NanoSIMS to investigate soil microenvironments, Advances in agronomy, Volume 121, Elsevier, p. 1-46.
- Newcomb, C. J., Qafoku, N. P., Grate, J. W., Bailey, V. L., and De Yoreo, J. J., 2017, Developing a molecular picture of soil organic matter-mineral interactions by quantifying organo-mineral binding: Nat Commun, v. 8, no. 1, p. 396.
- Praetorius, A., Gundlach-Graham, A., Goldberg, E., Fabienke, W., Navratilova, J., Gondikas, A., Kaegi, R., Günther, D., Hofmann, T., and von der Kammer, F., 2017, Single-particle multi-element fingerprinting (spMEF) using inductively-coupled plasma time-of-flight mass spectrometry (ICP-TOFMS) to identify engineered nanoparticles against the elevated natural background in soils: Environmental Science: Nano, v. 4, no. 2, p. 307-314.
- Qafoku, N. P., 2010, Terrestrial Nanoparticles and Their Controls on Soil-/Geo-Processes and Reactions, p. 33-91.
- Qafoku, N. P., 2015, Climate-change effects on soils: accelerated weathering, soil carbon, and elemental cycling, Advances in Agronomy, Volume 131, Elsevier, p. 111-172.
- Schindler, M., and Hochella Jr, M. F., 2016, Nanomineralogy as a new dimension in understanding elusive geochemical processes in soils: The case of low-solubility-index elements: Geology, v. 44, no. 7, p. 515-518.
- M. Schindler, M. F. Hochella Jr, Soil memory in mineral surface coatings: Environmental processes recorded at the nanoscale. Geology 43, 415-418 (2015).
- Schroeder, P. A., and Shiflet, J., 2000, Ti-bearing phases in the Huber Formation, an east Georgia kaolin deposit: Clays and Clay Minerals, v. 48, no. 2, p. 151-158.
- Schroeder, P. A., 2018, Clays in the Critical Zone, Cambridge University Press, 254 p.
Clays and clay minerals are the most abundant natural reactive solids on the Earth's surface. This comprehensive review considers clay science in the context of the Critical Zone - the Earth's permeable near-surface layer. Providing information on clays and clay minerals related to geological, biological and material sciences in the Critical Zone, it's well suited for graduate students and researchers interested in clay science, and environmental and soil mineralogy. The book starts with an introduction to clays and clay minerals, their historic background, and a review of how clay science impacts the Critical Zone. Examples and applications demonstrate how clays regulate habitats and determine the availability of other resources. These examples are supported by quantitative field data, including numerical and graphical depictions of clay and clay mineral occurrences. The book concludes by covering Critical Zone clay geochemistry and clay sequences, including the industrial, synthetic medical and extra-terrestrial world of clay science.
- Tingle, T. N., Borch, R. S., Hochella Jr, M. F., Becker, C. H., and Walker, W. J., 1993, Characterization of lead on mineral surfaces in soils contaminated by mining and smelting: Applied surface science, v. 72, no. 4, p. 301-306.
Nanoparticles and Chemical Weathering
- Gislason, S. R., Oelkers, E. H., Eiriksdottir, E. S., Kardjilov, M. I., Gisladottir, G., Sigfusson, B., Snorrason, A., Elefsen, S., Hardardottir, J., Torssander, P., and Oskarsson, N., 2009, Direct evidence of the feedback between climate and weathering: Earth and Planetary Science Letters, v. 277, no. 1-2, p. 213-222.
- Hellmann, R., Eggleston, C. M., Hochella Jr, M. F., and Crerar, D. A., 1990, The formation of leached layers on albite surfaces during dissolution under hydrothermal conditions: Geochimica et Cosmochimica Acta, v. 54, no. 5, p. 1267-1281.
- Hellmann, R., Wirth, R., Daval, D., Barnes, J.-P., Penisson, J.-M., Tisserand, D., Epicier, T., Florin, B., and Hervig, R. L., 2012, Unifying natural and laboratory chemical weathering with interfacial dissolution–reprecipitation: A study based on the nanometer-scale chemistry of fluid–silicate interfaces: Chemical Geology, v. 294, p. 203-216.
- Inskeep, W. P., Nater, E. A., Bloom, P. R., Vandervoort, D. S., and Erich, M. S., 1991, Characterization of laboratory weathered labradorite surfaces using X-ray photoelectron spectroscopy and transmission electron microscopy: Geochimica et Cosmochimica Acta, v. 55, no. 3, p. 787-800.
- Mogk, D. W., and Locke Iii, W. W., 1988, Application of auger electron spectroscopy (AES) to naturally weathered hornblende: Geochimica et Cosmochimica Acta, v. 52, no. 10, p. 2537-2542.
- Mogk, D. W., 1990, Application of Auger electron spectroscopy to studies of chemical weathering: Reviews of Geophysics, v. 28, no. 4, p. 337-356.
- Muir, I. J., Bancroft, G. M., Shotyk, W., and Nesbitt, H. W., 1990, A SIMS and XPS study of dissolving plagioclase: Geochimica et Cosmochimica Acta, v. 54, no. 8, p. 2247-2256.
- Ruiz-Agudo, E., Putnis, C. V., Rodriguez-Navarro, C., and Putnis, A., 2012, Mechanism of leached layer formation during chemical weathering of silicate minerals: Geology, v. 40, no. 10, p. 947-950.
- Ruiz-Agudo, E., King, H. E., Patiño-López, L. D., Putnis, C. V., Geisler, T., Rodriguez-Navarro, C., and Putnis, A., 2016, Control of silicate weathering by interface-coupled dissolution-precipitation processes at the mineral-solution interface: Geology, v. 44, no. 7, p. 567-570.
- Shotyk, W., and Metson, J. B., 1994, Secondary ion mass spectrometry (SIMS) and its application to chemical weathering: Reviews of Geophysics, v. 32, no. 2, p. 197-220.
Nanoparticles and Metals/Ore Deposits/Acid Mine Drainage
- De Corte, S., Hennebel, T., De Gusseme, B., Verstraete, W., and Boon, N., 2012, Bio-palladium: from metal recovery to catalytic applications: Microbial Biotechnology, v. 5, no. 1, p. 5-17.
- Deditius, A. P., Utsunomiya, S., Reich, M., Kesler, S. E., Ewing, R. C., Hough, R., and Walshe, J., 2011, Trace metal nanoparticles in pyrite: Ore Geology Reviews, v. 42, no. 1, p. 32-46.
- Deditius, A. P., Utsunomiya, S., Renock, D., Ewing, R. C., Ramana, C. V., Becker, U., and Kesler, S. E., 2008, A proposed new type of arsenian pyrite: Composition, nanostructure and geological significance: Geochimica et Cosmochimica Acta, v. 72, no. 12, p. 2919-2933
- Espana, J. S., Pamo, E. L., Santofimia, E., Aduvire, O., Reyes, J., and Barettino, D., 2005, Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): geochemistry, mineralogy and environmental implications: Applied geochemistry, v. 20, no. 7, p. 1320-1356.
- Glover, R. D., Miller, J. M., and Hutchison, J. E., 2011, Generation of metal nanoparticles from silver and copper objects: nanoparticle dynamics on surfaces and potential sources of nanoparticles in the environment: ACS nano, v. 5, no. 11, p. 8950-8957.
- Hamilton, J. L., Wilson, S. A., Morgan, B., Turvey, C. C., Paterson, D. J., MacRae, C., McCutcheon, J., and Southam, G., 2016, Nesquehonite sequesters transition metals and CO 2 during accelerated carbon mineralisation: International Journal of Greenhouse Gas Control, v. 55, p. 73-81.
- Hochella Jr, M. F., Kasama, T., Putnis, A., Putnis, C. V., and Moore, J. N., 2005, Environmentally important, poorly crystalline Fe/Mn hydrous oxides: Ferrihydrite and a possibly new vernadite-like mineral from the Clark Fork River Superfund Complex: American Mineralogist, v. 90, no. 4, p. 718-724.
- Hochella, M. F., Moore, J. N., Putnis, C. V., Putnis, A., Kasama, T., and Eberl, D. D., 2005, Direct observation of heavy metal-mineral association from the Clark Fork River Superfund Complex: Implications for metal transport and bioavailability: Geochimica et Cosmochimica Acta, v. 69, no. 7, p. 1651-1663.
- Mikhlin, Y., Romanchenko, A., Likhatski, M., Karacharov, A., Erenburg, S., and Trubina, S., 2011, Understanding the initial stages of precious metals precipitation: Nanoscale metallic and sulfidic species of gold and silver on pyrite surfaces: Ore Geology Reviews, v. 42, no. 1, p. 47-54.
- Olías, M., Cánovas, C. R., Nieto, J. M., and Sarmiento, A. M., 2006, Evaluation of the dissolved contaminant load transported by the Tinto and Odiel rivers (South West Spain): Applied Geochemistry, v. 21, no. 10, p. 1733-1749.
- Parnell, J., Perez, M., Armstrong, J., Bullock, L., Feldmann, J., and Boyce, A. J., 2018, Geochemistry and metallogeny of Neoproterozoic pyrite in oxic and anoxic sediments: Geochemical Perspectives Letters, p. 12-16.
- Schindler, M., Berti, D., and Hochella, M. F., 2017, Previously unknown mineral-nanomineral relationships with important environmental consequences: The case of chromium release from dissolving silicate minerals: American Mineralogist, v. 102, no. 10, p. 2142-2145.
- Plathe, K. L., von der Kammer, F., Hassellöv, M., Moore, J. N., Murayama, M., Hofmann, T., and Hochella, M. F., 2013, The role of nanominerals and mineral nanoparticles in the transport of toxic trace metals: Field-flow fractionation and analytical TEM analyses after nanoparticle isolation and density separation: Geochimica et Cosmochimica Acta, v. 102, p. 213-225.
Nanoparticles and Fault Mechanics
- Dor, O., Ben-Zion, Y., Rockwell, T. K., and Brune, J., 2006, Pulverized rocks in the Mojave section of the San Andreas Fault Zone: Earth and Planetary Science Letters, v. 245, no. 3-4, p. 642-654.
- Verberne, B. A., Plümper, O., de Winter, D. A. M., and Spiers, C. J., 2014, Superplastic nanofibrous slip zones control seismogenic fault friction: Science, v. 346, no. 6215, p. 1342-1344.
- Wilson, B., Dewers, T., Reches, Z. e., and Brune, J., 2005, Particle size and energetics of gouge from earthquake rupture zones: Nature, v. 434, no. 7034, p. 749.
Extraterrestrial Nanoparticles
- Becker, L., Poreda, R. J., and Bunch, T. E., 2000, Fullerenes: An extraterrestrial carbon carrier phase for noble gases: Proceedings of the National Academy of Sciences, v. 97, no. 7, p. 2979-2983.
- Dai, Z. R., Bradley, J. P., Joswiak, D. J., Brownlee, D. E., Hill, H. G. M., and Genge, M. J., 2002, Possible in situ formation of meteoritic nanodiamonds in the early solar system: Nature, v. 418, no. 6894,
- Mackinnon, I. D. R., and Mogk, D. W., 1985, Surface sulfur measurements on stratospheric particles: Geophysical Research letters, v. 12, no. 2, p. 93-96.
- Stadermann, F. J., Floss, C., Bose, M., and Lea, A. S., 2009, The use of Auger spectroscopy for the in situ elemental characterization of sub‐micrometer presolar grains: Meteoritics & Planetary Science, v. 44, no. 7, p. 1033-1049.
- Tingle, T. N., Becker, Christopher H., Malhotra, Ripudaman, 1991, Organic compounds in the Murchison and Allende carbonaceous chondrites studied by photoionization mass spectrometry Meteoritics v. 26, no. 2, p. 117-127.
- Verchovsky, A. B., Fisenko, A. V., Semjonova, L. F., Bridges, J., Lee, M. R., and Wright, I. P., 2006, Nanodiamonds from AGB stars: A new type of presolar grain in meteorites: The Astrophysical Journal, v. 651, no. 1, p. 481.
Nanoparticles and the Origin of Life (Mineral Templates that Organize Complex Organic Compounds) and Evolution of Life/Earth System
- Cleaves II, H. J., Scott, A. M., Hill, F. C., Leszczynski, J., Sahai, N., and Hazen, R., 2012, Mineral–organic interfacial processes: potential roles in the origins of life: Chemical Society Reviews, v. 41, no. 16, p. 5502-5525.
- Ferris, J. P., and Ertem, G., 1992, Oligomerization of ribonucleotides on montmorillonite: reaction of the 5'-phosphorimidazolide of adenosine: Science, v. 257, no. 5075, p. 1387-1389.
- Forterre, P., and Gribaldo, S., 2007, The origin of modern terrestrial life: HFSP Journal, v. 1, no. 3, p. 156-168.
- Hanczyc, M. M., Mansy, S. S., and Szostak, J. W., 2007, Mineral surface directed membrane assembly: Origins of Life and Evolution of Biospheres, v. 37, no. 1, p. 67-82.
- Metch, J. W., Burrows, N. D., Murphy, C. J., Pruden, A., and Vikesland, P. J., 2018, Metagenomic analysis of microbial communities yields insight into impacts of nanoparticle design: Nature nanotechnology, p. 1.
- Oleson, T. A., Sahai, N., and Pedersen, J. A., 2010, Electrostatic effects on deposition of multiple phospholipid bilayers at oxide surfaces: J Colloid Interface Sci, v. 352, no. 2, p. 327-336.
- Rasmussen, B., Krapež, B., Muhling, J. R., and Suvorova, A., 2015, Precipitation of iron silicate nanoparticles in early Precambrian oceans marks Earth's first iron age: Geology, v. 43, no. 4, p. 303-306.
- Sahai, N., Kaddour, H., and Dalai, P., 2016, The transition from geochemistry to biogeochemistry: Elements, v. 12, no. 6, p. 389-394.
- Sahai, N., Kaddour, H., Dalai, P., Wang, Z., Bass, G., and Gao, M., 2017, Mineral surface chemistry and nanoparticle-aggregation control membrane self-assembly: Scientific reports, v. 7, p. 43418.
- Xu, J., Stevens, M. J., Oleson, T. A., Last, J. A., and Sahai, N., 2009, Role of Oxide Surface Chemistry and Phospholipid Phase on Adsorption and Self-Assembly: Isotherms and Atomic Force Microscopy: The Journal of Physical Chemistry C, v. 113, no. 6, p. 2187-2196.
- Xu, J., Campbell, J. M., Zhang, N., Hickey, W. J., and Sahai, N., 2012, Did mineral surface chemistry and toxicity contribute to evolution of microbial extracellular polymeric substances?: Astrobiology, v. 12, no. 8, p. 785-798.
Nanoparticles and Impacts on Biota/Biota-Nanoparticle Interactions
- Canesi, L., and Corsi, I., 2016, Effects of nanomaterials on marine invertebrates: Sci Total Environ, v. 565, p. 933-940.
- Chae, Y., Kim, D., Kim, S. W., and An, Y.-J., 2018, Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain: Scientific reports, v. 8, no. 1, p. 284.
- Colman, B. P., Arnaout, C. L., Anciaux, S., Gunsch, C. K., Hochella, M. F., Jr., Kim, B., Lowry, G. V., McGill, B. M., Reinsch, B. C., Richardson, C. J., Unrine, J. M., Wright, J. P., Yin, L., and Bernhardt, E. S., 2013, Low Concentrations of Silver Nanoparticles in Biosolids Cause Adverse Ecosystem Responses under Realistic Field Scenario: PLOS ONE, v. 8, no. 2, p. e57189.
- Lee, W.-T., Wu, Y.-N., Chen, Y.-H., Wu, S.-R., Shih, T.-M., Li, T.-J., Yang, L.-X., Yeh, C.-S., Tsai, P.-J., and Shieh, D.-B., 2017, Octahedron Iron Oxide Nanocrystals Prohibited Clostridium difficile Spore Germination and Attenuated Local and Systemic Inflammation: Scientific reports, v. 7, no. 1, p. 8124.
- Li, M., Toner, B. M., Baker, B. J., Breier, J. A., Sheik, C. S., and Dick, G. J., 2014, Microbial iron uptake as a mechanism for dispersing iron from deep-sea hydrothermal vents: Nature communications, v. 5, p. 3192.
- Li, W., Xu, L., Liu, X., Zhang, J., Lin, Y., Yao, X., Gao, H., Zhang, D., Chen, J., and Wang, W., 2017, Air pollution–aerosol interactions produce more bioavailable iron for ocean ecosystems: Science advances, v. 3, no. 3, p. e1601749.
- Lower, S. K., Hochella, M. F., and Beveridge, T. J., 2001, Bacterial Recognition of Mineral Surfaces: Nanoscale Interactions Between Shewanella and α-FeOOH: Science, v. 292, no. 5520, p. 1360-1363.
- Luther, G. W., and Rickard, D. T., 2005, Metal Sulfide Cluster Complexes and their Biogeochemical Importance in the Environment: Journal of Nanoparticle Research, v. 7, no. 4-5, p. 389-407.
- Manceau, A., Wang, J., Rovezzi, M., Glatzel, P., and Feng, X., 2018, Biogenesis of Mercury-Sulfur Nanoparticles in Plant Leaves from Atmospheric Gaseous Mercury: Environ Sci Technol, v. 52, no. 7, p. 3935-3948.
- Metch, J. W., Burrows, N. D., Murphy, C. J., Pruden, A., and Vikesland, P. J., 2018, Metagenomic analysis of microbial communities yields insight into impacts of nanoparticle design: Nature nanotechnology, p. 1.
- Mueller, C. W., Weber, P. K., Kilburn, M. R., Hoeschen, C., Kleber, M., and Pett-Ridge, J., 2013, Advances in the analysis of biogeochemical interfaces: NanoSIMS to investigate soil microenvironments, Advances in agronomy, Volume 121, Elsevier, p. 1-46.
- Tang, R., Wang, L., and Nancollas, G. H., 2004, Size-effects in the dissolution of hydroxyapatite: an understanding of biological demineralization: Journal of Materials Chemistry, v. 14, no. 14, p. 2341-2346.
Biogenic Formation of Nanoparticles
- Coker, V. S., Telling, N. D., van der Laan, G., Pattrick, R. A. D., Pearce, C. I., Arenholz, E., Tuna, F., Winpenny, R. E. P., and Lloyd, J. R., 2009, Harnessing the extracellular bacterial production of nanoscale cobalt ferrite with exploitable magnetic properties: ACS nano, v. 3, no. 7, p. 1922-1928.
- De Corte, S., Hennebel, T., De Gusseme, B., Verstraete, W., and Boon, N., 2012, Bio-palladium: from metal recovery to catalytic applications: Microbial Biotechnology, v. 5, no. 1, p. 5-17.
- Egglseder, M. S., Cruden, A. R., Tomkins, A. G., Wilson, S. A., and Langendam, A. D., 2018, Colloidal origin of microbands in banded iron formations: Geochemical Perspectives Letters, p. 43-49.
- Gorby, Y. A., Yanina, S., McLean, J. S., Rosso, K. M., Moyles, D., Dohnalkova, A., Beveridge, T. J., Chang, I. S., Kim, B. H., Kim, K. S., Culley, D. E., Reed, S. B., Romine, M. F., Saffarini, D. A., Hill, E. A., Shi, L., Elias, D. A., Kennedy, D. W., Pinchuk, G., Watanabe, K., Ishii, S., Logan, B., Nealson, K. H., and Fredrickson, J. K., 2006, Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms: Proc Natl Acad Sci U S A, v. 103, no. 30, p. 11358-11363.
- Huang, J., Lin, L., Sun, D., Chen, H., Yang, D., and Li, Q., 2015, Bio-inspired synthesis of metal nanomaterials and applications: Chem Soc Rev, v. 44, no. 17, p. 6330-6374.
- Kandianis, M. T., Fouke, B. W., Johnson, R. W., Veysey, J., and Inskeep, W. P., 2008, Microbial biomass: A catalyst for CaCO3 precipitation in advection-dominated transport regimes: Geological Society of America Bulletin, v. 120, no. 3-4, p. 442-450.
- Korbekandi, H., Iravani, S., and Abbasi, S., 2009, Production of nanoparticles using organisms: Critical Reviews in Biotechnology, v. 29, no. 4, p. 279-306.
- Kyle, J. E., Eydal, H. S., Ferris, F. G., and Pedersen, K., 2008, Viruses in granitic groundwater from 69 to 450 m depth of the Aspo hard rock laboratory, Sweden: ISME J, v. 2, no. 5, p. 571-574.
- Lahr, R. H., and Vikesland, P. J., 2014, Surface-enhanced Raman spectroscopy (SERS) cellular imaging of intracellulary biosynthesized gold nanoparticles: ACS Sustainable Chemistry & Engineering, v. 2, no. 7, p. 1599-1608.
- Metch, J. W., Burrows, N. D., Murphy, C. J., Pruden, A., and Vikesland, P. J., 2018, Metagenomic analysis of microbial communities yields insight into impacts of nanoparticle design: Nature nanotechnology, p. 1.
- Mittal, A. K., Chisti, Y., and Banerjee, U. C., 2013, Synthesis of metallic nanoparticles using plant extracts: Biotechnology Advances, v. 31, no. 2, p. 346-356.
- Perri, E., Tucker, M. E., Słowakiewicz, M., Whitaker, F., Bowen, L., Perrotta, I. D., and Della Porta, G., 2017, Carbonate and silicate biomineralization in a hypersaline microbial mat (Mesaieed sabkha, Qatar): Roles of bacteria, extracellular polymeric substances and viruses: Sedimentology.
- Schrofel, A., Kratosova, G., Safarik, I., Safarikova, M., Raska, I., and Shor, L. M., 2014, Applications of biosynthesized metallic nanoparticles - a review: Acta Biomater, v. 10, no. 10, p. 4023-4042.
- Xie, J., Chen, K., and Chen, X., 2009, Production, modification and bio-applications of magnetic nanoparticles gestated by magnetotactic bacteria: Nano research, v. 2, no. 4, p. 261-278.
Nanoscience and Human Health--NanoToxicology
Nanoparticles and Diagnosis, Treatment, Drug Delivery

Long-term exposure to coal dust causes the illness known as black lung. Normal lung (left) and affected lung (right). Image from NIOSH, www.cdc.gov/niosh
![[reuse info]](/images/information_16.png)
Provenance: CDC/NIOSH, in the public domain; mage from NIOSH, www.cdc.gov/niosh
Reuse: This item is in the public domain and maybe reused freely without restriction.
The National Institute of Health/National Cancer Instittue Division of Cancer Treatment and Diagnosis has a very robust
Cancer Imaging Program - nanodelivery Systems and Devices Branch. Check out their collection of
Nanotechnology Visuals Online, and the YouTube video on
How Nanotechnology is Changing the Way we Look at Cancer.
- Bertrand, N., Wu, J., Xu, X., Kamaly, N., and Farokhzad, O. C., 2014, Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology: Advanced drug delivery reviews, v. 66, p. 2-25.
- Brun, E., Barreau, F., Veronesi, G., Fayard, B., Sorieul, S., Chanéac, C., Carapito, C., Rabilloud, T., Mabondzo, A., and Herlin-Boime, N., 2014, Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia: Particle and fibre toxicology, v. 11, no. 1, p. 13.
- Garner, K. L., and Keller, A. A., 2014, Emerging patterns for engineered nanomaterials in the environment: a review of fate and toxicity studies: Journal of Nanoparticle Research, v. 16, no. 8.
- Geiser, M., and Kreyling, W. G., 2010, Deposition and biokinetics of inhaled nanoparticles: Particle and fibre toxicology, v. 7, no. 1, p. 2.
- Heath, J. R., 2015, Nanotechnologies for biomedical science and translational medicine: Proc Natl Acad Sci U S A, v. 112, no. 47, p. 14436-14443.
- Hochella Jr, M., Madden, A.S., 2005, Earth's Nano-Compartment for Toxic Metals: Elements, v. 1, no. 4, p. 199-203.
- Holden, P. A., Nisbet, R. M., Lenihan, H. S., Miller, R. J., Cherr, G. N., Schimel, J. P., and Gardea-Torresdey, J. L., 2012, Ecological nanotoxicology: integrating nanomaterial hazard considerations across the subcellular, population, community, and ecosystems levels: Accounts of chemical research, v. 46, no. 3, p. 813-822.
- Jain, P. K., Huang, X., El-Sayed, I. H., and El-Sayed, M. A., 2008, Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine: Accounts of chemical research, v. 41, no. 12, p. 1578-1586.
- Li, B., Ze, Y., Sun, Q., Zhang, T., Sang, X., Cui, Y., Wang, X., Gui, S., Tan, D., and Zhu, M., 2013, Molecular mechanisms of nanosized titanium dioxide–induced pulmonary injury in mice: PloS one, v. 8, no. 2, p. e55563.
- Oberdörster, G., Oberdörster, E., and Oberdörster, J., 2005, Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles: Environmental health perspectives, v. 113, no. 7, p. 823.
- Oberdörster, G., Maynard, A., Donaldson, K., Castranova, V., Fitzpatrick, J., Ausman, K., Carter, J., Karn, B., Kreyling, W., and Lai, D., 2005, Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy: Particle and fibre toxicology, v. 2, no. 1, p. 8.
- Pelaz, B., Alexiou, C., Alvarez-Puebla, R. A., Alves, F., Andrews, A. M., Ashraf, S., Balogh, L. P., Ballerini, L., Bestetti, A., and Brendel, C., 2017, Diverse applications of nanomedicine, ACS Publications.
- Rod, T. H., and Nørskov, J. K., 2002, The surface science of enzymes: Surface Science, v. 500, no. 1, p. 678-698.
- Seaton, A., Tran, L., Aitken, R., and Donaldson, K., 2009, Nanoparticles, human health hazard and regulation: Journal of the Royal Society Interface, p. rsif20090252.
- Sharma, V. K., Filip, J., Zboril, R., and Varma, R. S., 2015, Natural inorganic nanoparticles--formation, fate, and toxicity in the environment: Chem Soc Rev, v. 44, no. 23, p. 8410-8423.
- Shi, J., Votruba, A. R., Farokhzad, O. C., and Langer, R., 2010, Nanotechnology in drug delivery and tissue engineering: from discovery to applications: Nano Lett, v. 10, no. 9, p. 3223-3230.
- Slavin, Y. N., Asnis, J., Häfeli, U. O., and Bach, H., 2017, Metal nanoparticles: understanding the mechanisms behind antibacterial activity: Journal of nanobiotechnology, v. 15, no. 1, p. 65.
- Stone, V., Miller, M. R., Clift, M. J. D., Elder, A., Mills, N. L., Moller, P., Schins, R. P. F., Vogel, U., Kreyling, W. G., Alstrup Jensen, K., Kuhlbusch, T. A. J., Schwarze, P. E., Hoet, P., Pietroiusti, A., De Vizcaya-Ruiz, A., Baeza-Squiban, A., Teixeira, J. P., Tran, C. L., and Cassee, F. R., 2017, Nanomaterials Versus Ambient Ultrafine Particles: An Opportunity to Exchange Toxicology Knowledge: Environ Health Perspect, v. 125, no. 10, p. 106002.
- Vikesland, P. J., and Wigginton, K. R., 2010, Nanomaterial enabled biosensors for pathogen monitoring-a review: Environmental science & technology, v. 44, no. 10, p. 3656-3669.
- Vriens, H., Mertens, D., Regret, R., Lin, P., Locquet, J.-P., and Hoet, P., 2017, Case Study III: The Construction of a Nanotoxicity Database–The MOD-ENP-TOX Experience, Modelling the Toxicity of Nanoparticles, Springer, p. 325-344.
- Wang, A. Z., Langer, R., and Farokhzad, O. C., 2012, Nanoparticle Delivery of Cancer Drugs: Annual Review of Medicine, v. 63, no. 1, p. 185-198.
- World Health, O., 2016, Ambient air pollution: A global assessment of exposure and burden of disease.
- Xia, T., Kovochich, M., Brant, J., Hotze, M., Sempf, J., Oberley, T., Sioutas, C., Yeh, J. I., Wiesner, M. R., and Nel, A. E., 2006, Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm: Nano letters, v. 6, no. 8, p. 1794-1807.
Naniparticles in Food and Consumer Products
- Schoepf, J. J., Bi, Y., Kidd, J., Herckes, P., Hristovski, K., and Westerhoff, P., 2017, Detection and dissolution of needle-like hydroxyapatite nanomaterials in infant formula: NanoImpact, v. 5, p. 22-28.
- Vance, M. E., Kuiken, T., Vejerano, E. P., McGinnis, S. P., Hochella Jr, M. F., Rejeski, D., and Hull, M. S., 2015, Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory: Beilstein journal of nanotechnology, v. 6, p. 1769.
- Weir, A., Westerhoff, P., Fabricius, L., Hristovski, K., and Von Goetz, N., 2012, Titanium dioxide nanoparticles in food and personal care products: Environmental science & technology, v. 46, no. 4, p. 2242-2250.
- Yada, R. Y., Buck, N., Canady, R., DeMerlis, C., Duncan, T., Janer, G., Juneja, L., Lin, M., McClements, D. J., and Noonan, G., 2014, Engineered nanoscale food ingredients: evaluation of current knowledge on material characteristics relevant to uptake from the gastrointestinal tract: Comprehensive Reviews in Food Science and Food Safety, v. 13, no. 4, p. 730-744.
Metal NPs and Human Health
- Maher, B. A., Ahmed, I. A. M., Karloukovski, V., MacLaren, D. A., Foulds, P. G., Allsop, D., Mann, D. M. A., Torres-Jardón, R., and Calderon-Garciduenas, L., 2016, Magnetite pollution nanoparticles in the human brain: Proceedings of the National Academy of Sciences, v. 113, no. 39, p. 10797-10801.
- Sharma, V. K., Filip, J., Zboril, R., and Varma, R. S., 2015, Natural inorganic nanoparticles--formation, fate, and toxicity in the environment: Chem Soc Rev, v. 44, no. 23, p. 8410-8423.
Atmospheric NPs and Human Health/Respiration/Mortality
- Fubini, B., and Fenoglio, I., 2007, Toxic potential of mineral dusts: Elements, v. 3, no. 6, p. 407-414.
- Geiser, M., and Kreyling, W. G., 2010, Deposition and biokinetics of inhaled nanoparticles: Particle and fibre toxicology, v. 7, no. 1, p. 2.
- Landrigan, P. J., 2017, Air pollution and health: The Lancet Public Health, v. 2, no. 1, p. e4-e5.
- Lelieveld, J., Evans, J. S., Fnais, M., Giannadaki, D., and Pozzer, A., 2015, The contribution of outdoor air pollution sources to premature mortality on a global scale: Nature, v. 525, no. 7569, p. 367.
- Li, B., Ze, Y., Sun, Q., Zhang, T., Sang, X., Cui, Y., Wang, X., Gui, S., Tan, D., and Zhu, M., 2013, Molecular mechanisms of nanosized titanium dioxide–induced pulmonary injury in mice: PloS one, v. 8, no. 2, p. e55563.
- Madl, A. K., Plummer, L. E., Carosino, C., and Pinkerton, K. E., 2014, Nanoparticles, lung injury, and the role of oxidant stress: Annu Rev Physiol, v. 76, p. 447-465.
- Meldrum, K., Guo, C., Marczylo, E. L., Gant, T. W., Smith, R., and Leonard, M. O., 2017, Mechanistic insight into the impact of nanomaterials on asthma and allergic airway disease: Part Fibre Toxicol, v. 14, no. 1, p. 45.
- Miller, M. R., Raftis, J. B., Langrish, J. P., McLean, S. G., Samutrtai, P., Connell, S. P., Wilson, S., Vesey, A. T., Fokkens, P. H. B., and Boere, A. J. F., 2017, Inhaled nanoparticles accumulate at sites of vascular disease: ACS nano, v. 11, no. 5, p. 4542-4552.
- Nel, A., 2005, Air pollution-related illness: effects of particles: Science, v. 308, no. 5723, p. 804-806.
- Roberts, R. A., Shen, T., Allen, I. C., Hasan, W., DeSimone, J. M., and Ting, J. P. Y., 2013, Analysis of the murine immune response to pulmonary delivery of precisely fabricated nano-and microscale particles: PloS one, v. 8, no. 4, p. e62115.
- Tsai, C.-J., Huang, C.-Y., Chen, S.-C., Ho, C.-E., Huang, C.-H., Chen, C.-W., Chang, C.-P., Tsai, S.-J., and Ellenbecker, M. J., 2011, Exposure assessment of nano-sized and respirable particles at different workplaces: Journal of Nanoparticle Research, v. 13, no. 9, p. 4161-4172.
- Viitanen, A. K., Uuksulainen, S., Koivisto, A. J., Hameri, K., and Kauppinen, T., 2017, Workplace Measurements of Ultrafine Particles-A Literature Review: Ann Work Expo Health, v. 61, no. 7, p. 749-758.
- World Health, O., 2016, Ambient air pollution: A global assessment of exposure and burden of disease.
- Yang, W., Elankumaran, S., and Marr, L. C., 2012, Relationship between humidity and influenza A viability in droplets and implications for influenza's seasonality: PLoS One, v. 7, no. 10, p. e46789.
Nanoparticles and Environmental Hazards and Remediation
- Baalousha, M., Yang, Y., Vance, M. E., Colman, B. P., McNeal, S., Xu, J., Blaszczak, J., Steele, M., Bernhardt, E., and Hochella, M. F., 2016, Outdoor urban nanomaterials: the emergence of a new, integrated, and critical field of study: Science of The Total Environment, v. 557, p. 740-753.
- Bernhardt, E. S., Colman, B. P., Hochella, M. F., Cardinale, B. J., Nisbet, R. M., Richardson, C. J., and Yin, L., 2010, An Ecological Perspective on Nanomaterial Impacts in the Environment: Journal of environmental quality, v. 39, no. 6, p. 1954-1965.
- Burkhardt, E.-M., Akob, D. M., Bischoff, S., Sitte, J., Kostka, J. E., Banerjee, D., Scheinost, A. C., and Küsel, K., 2009, Impact of biostimulated redox processes on metal dynamics in an iron-rich creek soil of a former uranium mining area: Environmental science & technology, v. 44, no. 1, p. 177-183.
- Civeira, M. S., Ramos, C. G., Oliveira, M. L., Kautzmann, R. M., Taffarel, S. R., Teixeira, E. C., and Silva, L. F., 2016, Nano-mineralogy of suspended sediment during the beginning of coal rejects spill: Chemosphere, v. 145, p. 142-147.
- Dale, J. G., Cox, S. S., Vance, M. E., Marr, L. C., and Hochella Jr, M. F., 2017, Transformation of cerium oxide nanoparticles from a diesel fuel additive during combustion in a diesel engine: Environmental science & technology, v. 51, no. 4, p. 1973-1980.
- Dunphy Guzman, K. A., Taylor, M. R., and Banfield, J. F., 2006, Environmental risks of nanotechnology: National nanotechnology initiative funding, 2000− 2004, ACS Publications.
- Eckelman, M. J., and Graedel, T. E., 2007, Silver emissions and their environmental impacts: a multilevel assessment: Environmental science & technology, v. 41, no. 17, p. 6283-6289.
- Espana, J. S., Pamo, E. L., Santofimia, E., Aduvire, O., Reyes, J., and Barettino, D., 2005, Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): geochemistry, mineralogy and environmental implications: Applied geochemistry, v. 20, no. 7, p. 1320-1356.
- Glover, R. D., Miller, J. M., and Hutchison, J. E., 2011, Generation of metal nanoparticles from silver and copper objects: nanoparticle dynamics on surfaces and potential sources of nanoparticles in the environment: ACS nano, v. 5, no. 11, p. 8950-8957.
- Hanigan, D., Truong, L., Schoepf, J., Nosaka, T., Mulchandani, A., Tanguay, R., and Westerhoff, P., 2018, Trade-offs in Ecosystem Impacts from Nanomaterial versus Organic Chemical Ultraviolet Filters in Sunscreens: Water Research, v. 139, p. 281-290.
- Holbrook, R. D., Motabar, D., Quifiones, O., Stanford, B., Vanderford, B., and Moss, D., 2013, Titanium distribution in swimming pool water is dominated by dissolved species: Environmental Pollution, v. 181, p. 68-74.
- Keller, A. A., and Lazareva, A., 2014, Predicted releases of engineered nanomaterials: From global to regional to local: Environ. Sci. Tech. Letters, v. 1, no. 1, p. 65-70.
- Lapworth, D. J., Stolpe, B., Williams, P. J., Gooddy, D. C., and Lead, J. R., 2013, Characterization of suboxic groundwater colloids using a multi-method approach: Environ Sci Technol, v. 47, no. 6, p. 2554-2561.
- Lusby, G., Hall, C., and Reiners, J., 2015, Lead Contamination of Surface Soils in Philadelphia from Lead Smelters and Urbanization: Environmental Justice, v. 8, no. 1, p. 6-14.
- Mitrano, D. M., Mehrabi, K., Dasilva, Y. A. R., and Nowack, B., 2017, Mobility of metallic (nano)particles in leachates from landfills containing waste incineration residues: Environmental Science: Nano, v. 4, no. 2, p. 480-492.
- Niu, J., Rasmussen, P. E., Magee, R., and Nilsson, G., 2015, Spatial and temporal variability of incidental nanoparticles in indoor workplaces: impact on the characterization of point source exposures: Environ Sci Process Impacts, v. 17, no. 1, p. 98-109.
- Nowack, B., and Bucheli, T. D., 2007, Occurrence, behavior and effects of nanoparticles in the environment: Environmental Pollution, v. 150, no. 1, p. 5-22.
- Nowack, B., Ranville, J. F., Diamond, S., Gallego-Urrea, J. A., Metcalfe, C., Rose, J., Horne, N., Koelmans, A. A., and Klaine, S. J., 2012, Potential scenarios for nanomaterial release and subsequent alteration in the environment: Environ Toxicol Chem, v. 31, no. 1, p. 50-59.
- Pavía-Sanders, A., Zhang, S., Flores, J. A., Sanders, J. E., Raymond, J. E., and Wooley, K. L., 2013, Robust magnetic/polymer hybrid nanoparticles designed for crude oil entrapment and recovery in aqueous environments: ACS nano, v. 7, no. 9, p. 7552-7561. "...deployment of hybrid nanocomposites, such as these, could aid in environmental remediation efforts, including at oil spill sites, in particular, following the bulk recovery phase."
- Posner, L. N., and Pandis, S. N., 2015, Sources of ultrafine particles in the Eastern United States: Atmospheric Environment, v. 111, p. 103-112.
- Pourzahedi, L., Pandorf, M., Ravikumar, D., Zimmerman, J. B., Seager, T. P., Theis, T. L., Westerhoff, P., Gilbertson, L. M., and Lowry, G. V., 2018, Life cycle considerations of nano-enabled agrochemicals: are today's tools up to the task?: Environmental Science: Nano, v. 5, no. 5, p. 1057-1069.
- Reed, R. B., Ladner, D. A., Higgins, C. P., Westerhoff, P., and Ranville, J. F., 2012, Solubility of nano‐zinc oxide in environmentally and biologically important matrices: Environmental Toxicology and Chemistry, v. 31, no. 1, p. 93-99.
- Riquelme, M. V., Leng, W., Carzolio, M., Pruden, A., and Vikesland, P., 2017, Stable oligonucleotide-functionalized gold nanosensors for environmental biocontaminant monitoring: Journal of Environmental Sciences, v. 62, p. 49-59.
- Schindler, M., Berti, D., and Hochella, M. F., 2017, Previously unknown mineral-nanomineral relationships with important environmental consequences: The case of chromium release from dissolving silicate minerals: American Mineralogist, v. 102, no. 10, p. 2142-2145.
- Sen, R., and Chakrabarti, S., 2012, Nanoscience pursuits in mineral particles and their environmental implications: International Journal of Environmental Sciences, v. 2, no. 4, p. 2295.
- Smita, S., Gupta, S. K., Bartonova, A., Dusinska, M., Gutleb, A. C., and Rahman, Q., 2012, Nanoparticles in the environment: assessment using the causal diagram approach: Environmental Health, v. 11, no. 1, p. S13.
- Sun, T. Y., Gottschalk, F., Hungerbuhler, K., and Nowack, B., 2014, Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials: Environ Pollut, v. 185, p. 69-76.
- Tumolva, L., Park, J. Y., Kim, J. S., Miller, A. L., Chow, J. C., Watson, J. G., and Park, K., 2010, Morphological and Elemental Classification of Freshly Emitted Soot Particles and Atmospheric Ultrafine Particles using the TEM/EDS: Aerosol Science and Technology, v. 44, no. 3, p. 202-215.
- Viitanen, A. K., Uuksulainen, S., Koivisto, A. J., Hameri, K., and Kauppinen, T., 2017, Workplace Measurements of Ultrafine Particles-A Literature Review: Ann Work Expo Health, v. 61, no. 7, p. 749-758.
- Yang, Y., Vance, M., Tou, F., Tiwari, A., Liu, M., and Hochella, M. F., 2016, Nanoparticles in road dust from impervious urban surfaces: distribution, identification, and environmental implications: Environmental Science: Nano, v. 3, no. 3, p. 534-544.
Discovery and Analysis of Engineered Nanoparticles in the Earth System
- Laborda, F., Bolea, E., Cepriá, G., Gómez, M. T., Jiménez, M. S., Pérez-Arantegui, J., and Castillo, J. R., 2016, Detection, characterization and quantification of inorganic engineered nanomaterials: a review of techniques and methodological approaches for the analysis of complex samples: Analytica chimica acta, v. 904, p. 10-32.
- Praetorius, A., Gundlach-Graham, A., Goldberg, E., Fabienke, W., Navratilova, J., Gondikas, A., Kaegi, R., Günther, D., Hofmann, T., and von der Kammer, F., 2017, Single-particle multi-element fingerprinting (spMEF) using inductively-coupled plasma time-of-flight mass spectrometry (ICP-TOFMS) to identify engineered nanoparticles against the elevated natural background in soils: Environmental Science: Nano, v. 4, no. 2, p. 307-314.
- Pourzahedi, L., Pandorf, M., Ravikumar, D., Zimmerman, J. B., Seager, T. P., Theis, T. L., Westerhoff, P., Gilbertson, L. M., and Lowry, G. V., 2018, Life cycle considerations of nano-enabled agrochemicals: are today's tools up to the task?: Environmental Science: Nano, v. 5, no. 5, p. 1057-1069.
- Sun, T. Y., Gottschalk, F., Hungerbuhler, K., and Nowack, B., 2014, Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials: Environ Pollut, v. 185, p. 69-76.
- Walker, W. C., Bosso, C. J., Eckelman, M., Isaacs, J. A., and Pourzahedi, L., 2015, Integrating life cycle assessment into managing potential EHS risks of engineered nanomaterials: reviewing progress to date: Journal of Nanoparticle Research, v. 17, no. 8, p. 344.
Nanoparticle Plastics in the Earth System
- Alimi, O. S., Farner Budarz, J., Hernandez, L. M., and Tufenkji, N., 2018, Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport: Environ Sci Technol, v. 52, no. 4, p. 1704-1724.
- Dawson, A. L., Kawaguchi, S., King, C. K., Townsend, K. A., King, R., Huston, W. M., and Nash, S. M. B., 2018, Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill: Nature communications, v. 9, no. 1, p. 1001.
- Law, K. L., and Thompson, R. C., 2014, Microplastics in the seas: Science, v. 345, no. 6193, p. 144-145.
- Lebreton, L. C. M., Van Der Zwet, J., Damsteeg, J. W., Slat, B., Andrady, A., and Reisser, J., 2017, River plastic emissions to the world's oceans. Nat. Commun. 8, 15611.
- Mattsson, K., Hansson, L. A., and Cedervall, T., 2015, Nano-plastics in the aquatic environment: Environmental Science: Processes & Impacts, v. 17, no. 10, p. 1712-1721.
- Rochman, C. M., 2018, Microplastics research—from sink to source: Science, v. 360, no. 6384, p. 28-29.
- Silva, A. B., Bastos, A. S., Justino, C. I. L., da Costa, J. P., Duarte, A. C., and Rocha-Santos, T. A. P., 2018, Microplastics in the environment: Challenges in analytical chemistry-A review: Analytica chimica acta.
- Wright, S. L., and Kelly, F. J., 2017, Plastic and Human Health: A Micro Issue?: Environmental Science & Technology, v. 51, no. 12, p. 6634-6647.
Nanoparticles and Heavy Metals in the Earth System
- Cánovas, C. R., Olías, M., Nieto, J. M., Sarmiento, A. M., and Cerón, J. C., 2007, Hydrogeochemical characteristics of the Tinto and Odiel Rivers (SW Spain). Factors controlling metal contents: Science of the Total Environment, v. 373, no. 1, p. 363-382.
- Espana, J. S., Pamo, E. L., Santofimia, E., Aduvire, O., Reyes, J., and Barettino, D., 2005, Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): geochemistry, mineralogy and environmental implications: Applied geochemistry, v. 20, no. 7, p. 1320-1356.
- Hochella Jr, M. F., Kasama, T., Putnis, A., Putnis, C. V., and Moore, J. N., 2005, Environmentally important, poorly crystalline Fe/Mn hydrous oxides: Ferrihydrite and a possibly new vernadite-like mineral from the Clark Fork River Superfund Complex: American Mineralogist, v. 90, no. 4, p. 718-724.
- Lee, S., Shen, Z., and Xu, H., 2016, Study on nanophase iron oxyhydroxides in freshwater ferromanganese nodules from Green Bay, Lake Michigan, with implications for the adsorption of As and heavy metals: American Mineralogist, v. 101, no. 9, p. 1986-1995.
- Luther, G. W., and Rickard, D. T., 2005, Metal Sulfide Cluster Complexes and their Biogeochemical Importance in the Environment: Journal of Nanoparticle Research, v. 7, no. 4-5, p. 389-407.
- Mikhlin, Y., Romanchenko, A., Likhatski, M., Karacharov, A., Erenburg, S., and Trubina, S., 2011, Understanding the initial stages of precious metals precipitation: Nanoscale metallic and sulfidic species of gold and silver on pyrite surfaces: Ore Geology Reviews, v. 42, no. 1, p. 47-54.
- Mitrano, D. M., Mehrabi, K., Dasilva, Y. A. R., and Nowack, B., 2017, Mobility of metallic (nano)particles in leachates from landfills containing waste incineration residues: Environmental Science: Nano, v. 4, no. 2, p. 480-492.
- Olías, M., Cánovas, C. R., Nieto, J. M., and Sarmiento, A. M., 2006, Evaluation of the dissolved contaminant load transported by the Tinto and Odiel rivers (South West Spain): Applied Geochemistry, v. 21, no. 10, p. 1733-1749.
- Plathe, K. L., von der Kammer, F., Hassellöv, M., Moore, J. N., Murayama, M., Hofmann, T., and Hochella, M. F., 2013, The role of nanominerals and mineral nanoparticles in the transport of toxic trace metals: Field-flow fractionation and analytical TEM analyses after nanoparticle isolation and density separation: Geochimica et Cosmochimica Acta, v. 102, p. 213-225.
- Schindler, M., Berti, D., and Hochella, M. F., 2017, Previously unknown mineral-nanomineral relationships with important environmental consequences: The case of chromium release from dissolving silicate minerals: American Mineralogist, v. 102, no. 10, p. 2142-2145.
- Tingle, T. N., Borch, R. S., Hochella Jr, M. F., Becker, C. H., and Walker, W. J., 1993, Characterization of lead on mineral surfaces in soils contaminated by mining and smelting: Applied surface science, v. 72, no. 4, p. 301-306.
- Utsunomiya, S., Jensen, K. A., Keeler, G. J., and Ewing, R. C., 2004, Direct identification of trace metals in fine and ultrafine particles in the Detroit urban atmosphere: Environmental Science & Technology, v. 38, no. 8, p. 2289-2297.
Nanoparticles, Radionuclides and Nuclear Waste Containment
- Burkhardt, E.-M., Akob, D. M., Bischoff, S., Sitte, J., Kostka, J. E., Banerjee, D., Scheinost, A. C., and Küsel, K., 2009, Impact of biostimulated redox processes on metal dynamics in an iron-rich creek soil of a former uranium mining area: Environmental science & technology, v. 44, no. 1, p. 177-183.
- Ilton, E. S., Qafoku, N. P., Liu, C., Moore, D. A., and Zachara, J. M., 2008, Advective removal of intraparticle uranium from contaminated vadose zone sediments, Hanford, US: Environmental science & technology, v. 42, no. 5, p. 1565-1571.
- Johnson, C. A., Freyer, G., Fabisch, M., Caraballo, M. A., Küsel, K., and Hochella, M. F., 2014, Observations and assessment of iron oxide and green rust nanoparticles in metal-polluted mine drainage within a steep redox gradient: Environmental Chemistry, v. 11, no. 4, p. 377-391.
- Johnson, C. A., Murayama, M., Küsel, K., and Hochella Jr, M. F., 2015, Polycrystallinity of green rust minerals and their synthetic analogs: Implications for particle formation and reactivity in complex systems: American Mineralogist, v. 100, no. 10, p. 2091-2105.
- McKinley, J. P., Zachara, J. M., Wan, J., McCready, D. E., and Heald, S. M., 2007, Geochemical controls on contaminant uranium in vadose Hanford formation sediments at the 200 area and 300 area, Hanford Site, Washington: Vadose Zone Journal, v. 6, no. 4, p. 1004-1017.
- Novikov, A. P., Kalmykov, S. N., Utsunomiya, S., Ewing, R. C., Horreard, F., Merkulov, A., Clark, S. B., Tkachev, V. V., and Myasoedov, B. F., 2006, Colloid transport of plutonium in the far-field of the Mayak Production Association, Russia: Science, v. 314, no. 5799, p. 638-641.
- Penrose, W. R., Polzer, W. L., Essington, E. H., Nelson, D. M., and Orlandini, K. A., 1990, Mobility of plutonium and americium through a shallow aquifer in a semiarid region: Environmental science & technology, v. 24, no. 2, p. 228-234.
- Qafoku, N. P., Kukkadapu, R. K., McKinley, J. P., Arey, B. W., Kelly, S. D., Wang, C., Resch, C. T., and Long, P. E., 2009, Uranium in framboidal pyrite from a naturally bioreduced alluvial sediment: Environmental science & technology, v. 43, no. 22, p. 8528-8534.
- Wersin, P., Hochella, M. F., Persson, P., Redden, G., Leckie, J. O., and Harris, D. W., 1994, Interaction between aqueous uranium (VI) and sulfide minerals: Spectroscopic evidence for sorption and reduction: Geochimica et Cosmochimica Acta, v. 58, no. 13, p. 2829-2843.
- Zachara, J. M., Serne, J., Freshley, M., Mann, F., Anderson, F., Wood, M., Jones, T., and Myers, D., 2007, Geochemical processes controlling migration of tank wastes in Hanford's vadose zone: Vadose Zone Journal, v. 6, no. 4, p. 985-1003.
Nanoparticle Fullerenes--Natural, Engineered and Incidental
- Becker, L., Poreda, R. J., and Bunch, T. E., 2000, Fullerenes: An extraterrestrial carbon carrier phase for noble gases: Proceedings of the National Academy of Sciences, v. 97, no. 7, p. 2979-2983.
- Krätschmer, W., Lamb, L. D., Fostiropoulos, K., and Huffman, D. R., 1990, Solid C60: a new form of carbon: Nature, v. 347, no. 6291, p. 354.
- Tiwari, A. J., Ashraf-Khorassani, M., and Marr, L. C., 2016, C60 fullerenes from combustion of common fuels: Sci Total Environ, v. 547, p. 254-260.
Assessing Risk of Nanoparticles in the Environment
- Arnaldi, S., Ferrari, A., Magaudda, P., and Marin, F., 2014, Responsibility in nanotechnology development, Springer.
- Boverhof, D. R., Bramante, C. M., Butala, J. H., Clancy, S. F., Lafranconi, M., West, J., and Gordon, S. C., 2015, Comparative assessment of nanomaterial definitions and safety evaluation considerations: Regul Toxicol Pharmacol, v. 73, no. 1, p. 137-150.
- Corio, L., and Olson, K., 2015, The Need for Alternate PM2. 5 Emission Factors for Gas-Fired Combustion Units: Power, v. 159, no. 7, p. 51-54.
- Dunphy Guzman, K. A., Taylor, M. R., and Banfield, J. F., 2006, Environmental risks of nanotechnology: National nanotechnology initiative funding, 2000− 2004, ACS Publications.
- Hendren, C. O., Mesnard, X., Dröge, J., and Wiesner, M. R., 2011, Estimating production data for five engineered nanomaterials as a basis for exposure assessment, ACS Publications.
- Renn, O., and Roco, M. C., 2006, Nanotechnology and the need for risk governance: Journal of Nanoparticle Research, v. 8, no. 2, p. 153-191.
- Smita, S., Gupta, S. K., Bartonova, A., Dusinska, M., Gutleb, A. C., and Rahman, Q., 2012, Nanoparticles in the environment: assessment using the causal diagram approach: Environmental Health, v. 11, no. 1, p. S13.
- Wiesner, M. R., Lowry, G. V., Alvarez, P., Dionysiou, D., and Biswas, P., 2006, Assessing the risks of manufactured nanomaterials, ACS Publications.
- Wiesner, M. R., Lowry, G. V., Jones, K. L., Hochella, J., Michael F, Di Giulio, R. T., Casman, E., and Bernhardt, E. S., 2009, Decreasing uncertainties in assessing environmental exposure, risk, and ecological implications of nanomaterials, ACS Publications.
Some Societal Issues Related to Nanoscience
- Bainbridge, W. S., 2010, Governing Nanotechnology: Social, Ethical and Human Issues, Springer Handbook of Nanotechnology, Springer, p. 1867-1883.
- Bainbridge, W. S., 2013, Converging technologies for improving human performance: Nanotechnology, biotechnology, information technology and cognitive science, Springer Science & Business Media.
- Bürgi, B. R., and Pradeep, T., 2006, Societal implications of nanoscience and nanotechnology in developing countries: CURRENT SCIENCE-BANGALORE-, v. 90, no. 5, p. 645.
- Chen, H., Roco, M. C., Son, J., Jiang, S., Larson, C. A., and Gao, Q., 2013, Global nanotechnology development from 1991 to 2012: patents, scientific publications, and effect of NSF funding: Journal of nanoparticle research, v. 15, no. 9, p. 1951.
- Crow, M. M., and Sarewitz, D., 2001, Nanotechnology and societal transformation: Societal implications of nanoscience and nanotechnology, p. 45.
- Falinski, M. M., Plata, D. L., Chopra, S. S., Theis, T. L., Gilbertson, L. M., and Zimmerman, J. B., 2018, A framework for sustainable nanomaterial selection and design based on performance, hazard, and economic considerations: Nature nanotechnology.
- Godwin, H. A., Chopra, K., Bradley, K. A., Cohen, Y., Harthorn, B. H., Hoek, E. M. V., Holden, P., Keller, A. A., Lenihan, H. S., Nisbet, R. M., and Nel, A. E., 2009, The University of California Center for the Environmental Implications of Nanotechnology: Environmental Science & Technology, v. 43, no. 17, p. 6453-6457.
- Hendren, C. O., Lowry, G. V., Unrine, J. M., and Wiesner, M. R., 2015, A functional assay-based strategy for nanomaterial risk forecasting: Sci Total Environ, v. 536, p. 1029-1037.
- Hochella, M. F., 2002, Nanoscience and technology: the next revolution in the Earth sciences: Earth and Planetary Science Letters, v. 203, no. 2, p. 593-605.
- Hoover, E., Brown, P., Averick, M., Kane, A., and Hurt, R., 2009, Teaching small and thinking large: Effects of including social and ethical implications in an interdisciplinary nanotechnology course: Journal of Nano Education, v. 1, no. 1, p. 86-95.
- Hullmann, A. (2008). European activities in the field of ethical, legal and social aspects (ELSA) and governance of nanotechnology. DG Research, Brussels: European Commission.
- Kumar, P., Robins, A., Vardoulakis, S., and Britter, R., 2010, A review of the characteristics of nanoparticles in the urban atmosphere and the prospects for developing regulatory controls: Atmospheric Environment, v. 44, no. 39, p. 5035-5052.
- Liu, Y., Zhou, G., Liu, K., and Cui, Y., 2017, Design of complex nanomaterials for energy storage: past success and future opportunity: Accounts of chemical research, v. 50, no. 12, p. 2895-2905.
- Madden, A. S., Knefel, A. M. C., Grady, J. R., Glasson, G. E., Hochella Jr, M. H., Eriksson, S. C., Bank, T. L., Cecil, K., Green, A. M., and Hurst, A. N., 2007, Nano2earth: Incorporating cutting-edge research into secondary education through scientist-educator partnerships: Journal of Geoscience Education, v. 55, no. 5, p. 402-412.
- Roco, C., and Bainbridge, W. S., 2002, Converging Technologies for Improving Human Performance NANOTECHNOLOGY, BIOTECHNOLOGY, INFORMATION TECHNOLOGY AND COGNITIVE SCIENCE.
- Roco, M. C., 2003, Broader societal issues of nanotechnology: Journal of Nanoparticle Research, v. 5, no. 3-4, p. 181-189.
- Roco, M. C., 2011, The long view of nanotechnology development: the National Nanotechnology Initiative at 10 years, Springer.
- Roco, M. C., and Bainbridge, W. S., 2002, Converging technologies for improving human performance: Integrating from the nanoscale: Journal of nanoparticle research, v. 4, no. 4, p. 281-295.
- Roco, M. C., and Bainbridge, W. S., 2005, Societal implications of nanoscience and nanotechnology: Maximizing human benefit: Journal of Nanoparticle Research, v. 7, no. 1, p. 1-13.
- Roco, M. C., Harthorn, B., Guston, D., and Shapira, P., 2011, Innovative and responsible governance of nanotechnology for societal development, Nanotechnology Research Directions for Societal Needs in 2020, Springer, p. 561-617.
- Scheufele, D. A., and Brossard, D., 2008, Nanotechnology as a Moral Issue? Religion and Science in the US: NANOTECHNOLOGY, v. 21, no. 1.
- Seaton, A., Tran, L., Aitken, R., and Donaldson, K., 2009, Nanoparticles, human health hazard and regulation: Journal of the Royal Society Interface, p. rsif20090252.
- Sweeney, A. E., 2006, Social and ethical dimensions of nanoscale science and engineering research: Science and Engineering Ethics, v. 12, no. 3, p. 435-464.
- Tenner, E., 2001, Nanotechnology and unintended consequences: Societal Implications of Nanoscience and Nanotechnology, v. 50, no. 2, p. 311.
- Vance, M. E., Kuiken, T., Vejerano, E. P., McGinnis, S. P., Hochella, M. F., Jr., Rejeski, D., and Hull, M. S., 2015, Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory: Beilstein J Nanotechnol, v. 6, p. 1769-1780.
Ethics and Nanoscience
Centers on Ethics and Nanoscience
- The Center for Nanotechnology and Society--Arizona State University; resources on Research, Education, and Outreach; "CNS-ASU develops programs that integrate academic and societal concerns in order to better understand how to govern new technologies, from their birth in the laboratory to their entrance into the mainstream. Mission CNS-ASU's mission is to:Research the societal implications of nanotechnology and emerging technologies.Train an interdisciplinary community of scholars with new insights into the societal dimensions of emerging technologies.Engage the public, policy-makers, business leaders and researchers in dialogues about the goals and implications of emerging technologies.Partner with cutting-edge laboratories to cultivate greater reflexiveness in research, development, education and policy.
- NSF Center for Nanotechnology in Society at UCSB--University of California, Santa Barbara; "The NSF Center for Nanotechnology in Society at UCSB serves as a national research and education center, a network hub among researchers and educators concerned with societal issues concerning nanotechnologies, and a resource base for studying these issues in the US and abroad. The Center addresses education for a new generation of social science and nanoscience professionals, and it conducts research on the historical context of the nano-enterprise, on innovation processes and global diffusion of nanotech, and on risk perception and the public sphere. CNS-UCSB researchers address a linked set of social and environmental issues regarding the domestic US and global creation, development, commercialization, production, consumption, and control of specific kinds of nanoscale technologies."
- The Nanoethics Group--Cal State San Luis Obispo; excellent resources including exploration of The Issues.
- Ethics Instructional Resources--from the collections of nanoHub developed at Purdue University.
Journal Articles on Ethics and Nanoscience
- JOURNAL: Studies of New and Emerging Technologies--Journal published by Springer, online volumes from 2007-2018.
- Allhoff, F. (2009). On the autonomy and justification of nanoethics. In Nanotechnology & society (pp. 3-38). Springer, Dordrecht.
- Arnaldi, S., Ferrari, A., Magaudda, P., and Marin, F., 2014, Responsibility in nanotechnology development, Springer.
- Berne, R. W., 2005, Nanotalk: Conversations with scientists and engineers about ethics, meaning, and belief in the development of nanotechnology, CRC Press.
- Bürgi, B. R., and Pradeep, T., 2006, Societal implications of nanoscience and nanotechnology in developing countries: CURRENT SCIENCE-BANGALORE-, v. 90, no. 5, p. 645.
- Chen, H., Roco, M. C., Son, J., Jiang, S., Larson, C. A., and Gao, Q., 2013, Global nanotechnology development from 1991 to 2012: patents, scientific publications, and effect of NSF funding: Journal of nanoparticle research, v. 15, no. 9, p. 1951.
- Crow, M. M., and Sarewitz, D., 2001, Nanotechnology and societal transformation: Societal implications of nanoscience and nanotechnology, p. 45.
- David, K., and Thompson, P. B., 2011, What can nanotechnology learn from biotechnology?: social and ethical lessons for nanoscience from the debate over agrifood biotechnology and GMOs, Academic Press.
- Dunphy Guzman, K. A., Taylor, M. R., and Banfield, J. F., 2006, Environmental risks of nanotechnology: National nanotechnology initiative funding, 2000− 2004, ACS Publications.
- Ferrari, A. (2010). Developments in the debate on nanoethics: traditional approaches and the need for new kinds of analysis. NanoEthics, 4(1), 27-52.
- Fisher, E., 2007, The convergence of nanotechnology, policy, and ethics: Advances in Computers, v. 71, p. 273-296.
- Giese, B., Klaessig, F., Park, B., Kaegi, R., Steinfeldt, M., Wigger, H., Gleich, A., and Gottschalk, F., 2018, Risks, release and concentrations of engineered nanomaterial in the environment: Scientific reports, v. 8, no. 1, p. 1565.
- Godwin, H. A., Chopra, K., Bradley, K. A., Cohen, Y., Harthorn, B. H., Hoek, E. M. V., Holden, P., Keller, A. A., Lenihan, H. S., Nisbet, R. M., and Nel, A. E., 2009, The University of California Center for the Environmental Implications of Nanotechnology†: Environmental Science & Technology, v. 43, no. 17, p. 6453-6457.
- Hogle, Linda F., 2009, Science, Ethics, and the "Problems" of Governing Nanotechnologies: The Journal of Law, Medicine & Ethics, v. 37, no. 4, p. 749-758.
- Hoover, E., Brown, P., Averick, M., Kane, A., and Hurt, R., 2009, Teaching small and thinking large: Effects of including social and ethical implications in an interdisciplinary nanotechnology course: Journal of Nano Education, v. 1, no. 1, p. 86-95.
- Keiper, A., Nanoethics as a discipline--The New Atlantis Journal of Technology and Society.
- Laherto, A. (2010). An analysis of the educational significance of nanoscience and nanotechnology in scientific and technological literacy. Science Education International, 21(3), 160-175.
- Lead, J. R., Aruguete, D. M., and Hochella Jr, M. F., 2010, Manufactured nanoparticles in the environment: Environmental Chemistry, v. 7, no. 1, p. 1-2.
- McGinn, R. E. (2010). What's different, ethically, about nanotechnology?: foundational questions and answers. Nanoethics, 4(2), 115-128.
- Mnyusiwalla, A., Daar, A. S., and Singer, P. A., 2003, 'Mind the gap': science and ethics in nanotechnology: Nanotechnology, v. 14, no. 3, p. R9.
- National Science and Technology Council, C. o. T., Subcommittee on Nanoscale Science, and Engineering, a. T., 2014, National Nanotechnology Initiative Strategic Plan p. 88.
- Nordmann, A., and Rip, A., 2009, Mind the gap revisited: Nature nanotechnology, v. 4, no. 5, p. 273.
- Oberdörster, G., Maynard, A., Donaldson, K., Castranova, V., Fitzpatrick, J., Ausman, K., Carter, J., Karn, B., Kreyling, W., and Lai, D., 2005, Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy: Particle and fibre toxicology, v. 2, no. 1, p. 8.
- Rasch, P. J., Tilmes, S., Turco, R. P., Robock, A., Oman, L., Chen, C.-C. J., Stenchikov, G. L., and Garcia, R. R., 2008, An overview of geoengineering of climate using stratospheric sulphate aerosols: Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences, v. 366, no. 1882, p. 4007-4037.
- Renn, O., and Roco, M. C., 2006, Nanotechnology and the need for risk governance: Journal of Nanoparticle Research, v. 8, no. 2, p. 153-191.
- Scheufele, D. A., and Brossard, D., 2008, Nanotechnology as a Moral Issue? Religion and Science in the US: NANOTECHNOLOGY, v. 21, no. 1.
- Seaton, A., Tran, L., Aitken, R., and Donaldson, K., 2009, Nanoparticles, human health hazard and regulation: Journal of the Royal Society Interface, p. rsif20090252.
- Sweeney, A. E., 2006, Social and ethical dimensions of nanoscale science and engineering research: Science and Engineering Ethics, v. 12, no. 3, p. 435-464.
- Swierstra, T., and Rip, A., 2007, Nano-ethics as NEST-ethics: Patterns of Moral Argumentation About New and Emerging Science and Technology: NanoEthics, v. 1, no. 1, p. 3-20
- Temple, J., 2018, Will the world ever be ready for solar geoengineering?(vol 96, pg 28, 2018): Chemical & Engineering News, v. 96, no. 14, p. 5-5.
- Tenner, E., 2001, Nanotechnology and unintended consequences: Societal Implications of Nanoscience and Nanotechnology, v. 50, no. 2, p. 311.
- Van de Poel, I. (2008). How should we do nanoethics? A network approach for discerning ethical issues in nanotechnology. NanoEthics, 2(1), 25-38.
- Weil, V., 2001, Ethical issues in nanotechnology: Societal implications of nanoscience and nanotechnology, v. 193.
- Wiesner, M. R., Lowry, G. V., Alvarez, P., Dionysiou, D., and Biswas, P., 2006, Assessing the risks of manufactured nanomaterials, ACS Publications
Books on Ethics and Nanoscience
- Allhoff, F., Lin, P., Moor, J., and Weckert, J., 2007, Nanoethics: The Ethical and Social Implications of Nanotechnology, Wiley, 416 pp.
- Allhoff, F., & Lin, P. (Eds.). (2008). Nanotechnology & society: Current and emerging ethical issues. Springer Science & Business Media.
- Allhoff, F., Lin, P., & Moore, D. (2009). What is nanotechnology and why does it matter?: From science to ethics. John Wiley & Sons.
- Arnaldi, S., Ferrari, A., Magaudda, P., and Marin, F., 2014, Responsibility in nanotechnology development, Springer.
- Bainbridge, W. S., 2010, Governing Nanotechnology: Social, Ethical and Human Issues, Springer Handbook of Nanotechnology, Springer, p. 1867-1883.
- Berne, R. W., 2005, Nanotalk: Conversations with scientists and engineers about ethics, meaning, and belief in the development of nanotechnology, CRC Press.
- Lin, P., Moor, J. H., & Weckert, J. (2007). Nanoethics: the ethical and social implications of nanotechnology. John Wiley & Sons.
- Nanoethics ethics for technologies that converge at the nanoscale. 2007. Dordrecht: Springer Netherlands.
- O'Mathuna, D. P. (2009). Nanoethics: Big ethical issues with small technology. Bloomsbury Publishing.






![[creative commons]](/images/creativecommons_16.png)








![[reuse info]](/images/information_16.png)
