Laser-Induced Breakdown Spectroscopy (LIBS)
What is Laser-Induced Breakdown Spectroscopy (LIBS)
Laser-Induced Breakdown Spectroscopy (LIBS) is a rapid, portable, in situ atomic spectroscopy technique used to measure the concentration of major and trace elements in solid, liquid, or air samples, or to record the chemical signature (fingerprint) of a material. Each LIBS spectrum contains not only information about the concentrations of all naturally-occurring elements, but also some isotopic ratios and information about the atomic structure of the material. LIBS is a spot analysis technique, with laser ablation craters on the order of 30 - 400 µm diameter, depending on the laser wavelength, power, properties of the material itself, and how well the laser couples to the material.
Because LIBS is a spot analysis technique, it is possible to evaluate spatial changes in material composition and also to average shots taken from many different locations on the materials to obtain a bulk composition.
Fundamental Principles of Laser-Induced Breakdown Spectroscopy (LIBS)
A high-power laser pulse is used as an energy source to cause ablation of atoms from the sample surface and formation of a short-lived, high-temperature plasma. Plasma temperatures are generally hotter than 10,000 K with sufficient energy to cause excitation of electrons in outer orbitals. As the plasma cools, the excited electrons decay to lower-energy orbitals, emitting photons with wavelengths inversely proportional to the energy difference between the excited and base orbitals. There are many possible excited states and thus many emitted wavelengths for each element.
Molecules in the plasma also emit photons that are recorded as part of the LIBS spectrum. Molecules with different isotopic compositions (for instance, 11BO and 10BO) emit photons with slightly different wavelengths; measurement of isotopic ratios using this method is called Laser-Induced Molecular Isotopic Spectrometry (LAMIS; Russo et al., 2011).
Recent work (Serrano et al., 2015) suggests that molecular structure influences the emission of laser ablation plasmas. Different electron structures in organic materials result in wavelength shifts of molecular emissions.
Laser-Induced Breakdown Spectroscopy (LIBS) Instrumentation - How Does It Work?
Lasers
A variety of lasers are used in LIBS systems. The Nd:YAG laser operated at its fundamental wavelength of 1064 nm or at one of the harmonic wavelengths, typically 266 nm or 213 nm, is the most common laser used. Pulse widths are typically 6-15 ns, with repetition rates from single shots to >20 Hz. Single-pulse LIBS uses a single laser pulse to ablate and excite atoms. Double-pulse LIBS employs a second laser pulse, either perpendicular to or colinear with the first pulse to excite a higher proportion of atoms from the sample, thereby increasing peak intensities with no additional impact to the sample.
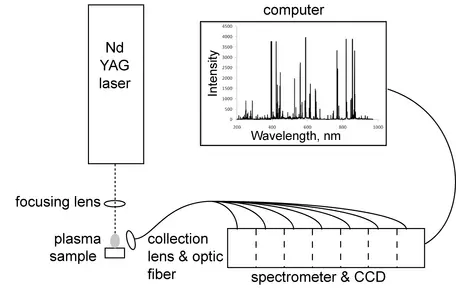
Light Collection
In most laboratory and portable LIBS systems, photons from the plasma are collected through a lens near the plasma and transmitted to the spectrometer via fiber optic. An alternative method is called stand-off LIBS, which is used in the ChemCam instrument package on NASA's Mars Science Laboratory Curiosity. The sample analyzed in stand-off LIBS is several meters from the instrument itself, and photon emission is captured by telescope and transmitted to the spectrometer by fiber optic.
Diffraction of Light to Create a Spectrum
Several types of spectrometers are uses in LIBS systems, most commonly echelle spectrographs. Spectrograph output is recorded on a CCD (charge-coupled device), much like a digital camera. In many cases, photon collection begins a few microseconds after the laser pulse to minimize collection of light emitted by non-quantum processes, such as kinetic interactions between electrons and atoms. The time between laser ablation and photon collection is called the Q-switch delay.Applications
LIBS has been applied to a wide range of applications because of it is a rapid and portable technique that requires no sample preparation that provides immediate, in situ analysis. It is even being employed robotically on Mars.
Applications fall into two broad categories: elemental analysis and chemical fingerprinting. Because every naturally-occurring element emits light in the wavelength range typically recorded (200 -1000 nm), it possible to simultaneously detect or analyze many elements. Elemental analysis of rocks, minerals, natural and contaminated soils, fingernails, food products, atmospheric particulate matter, and many more materials has been accomplished.
LIBS spectra are very useful for solving problems by using the spectra as chemical signatures, or fingerprints, of materials and comparing them to a database of spectra of known materials. This idea has been applied to the provenance of gems and conflict minerals, the identity of bacteria in blood, the engineering properties of highway aggregates, quality control in industrial processes such as heat treatments, and many other situations. Materialytics, a LIBS-based material science company, has coined the term Quantagenetics® to indicate that every material has a unique chemical and structural signature that is captured in a LIBS spectrum. The Quantagenetics® signature can be used to discover the origin and history of the material through comparison with known samples.
Strengths and Limitations of Laser-Induced Breakdown Spectroscopy (LIBS)?
Strengths
LIBS occupies a unique niche in the field of analytical techniques. Its strengths are that analysis is rapid and requires essentially no sample preparation. It is also portable in handheld units that are approximately the size of a cordless drill. LIBS is very sensitive to the light elements, including H, Li, and Be. In addition, LIBS spectra contain detailed compositional information about the material, making the technique ideal for correlation and provenance studies.
Limitations
LIBS has three major limitations. First, there are significant shot-to-shot variations in intensity, largely because the laser doesn't interact with the sample in exactly the same manner for every laser pulse. This is often overcome by averaging many spectra of the same material. Second, elemental analysis using univariate or multivariate calibrations does not yield the accuracy or precision of more traditional techniques such as ICP-MS or XRF. Finally, as with any spectroscopic technique, the spectra produced by one LIBS instrument are unique to that instrument, and the same sample analyzed on a similar instrument will be slightly different. When using LIBS spectra to do provenance studies, these inter-instrument differences are sufficiently large to prevent combining data sets from more than one instrument.
User's Guide - Sample Collection and Preparation
No sample preparation is necessary for LIBS analysis; it is a point and shoot technique. However, LIBS analyzes only the surface of materials, so it is important to make sure that a fresh, representative surface is analyzed. Theoretically, there is no limitation in the size or shape of material analyzed. However, many desktop systems have a sample chamber enclosed by eye-safe plastic that might constrain maximum sample size. Minimum sample size is determined by the spot size, which is controlled largely by laser wavelength and power.
Data Collection, Results and Presentation
Elemental analysis with LIBS spectra has been performed using univariate analysis (using only one peak) and multivariate analysis (using several peaks of the same element or the entire LIBS spectrum). Multivariate analysis is commonly accomplished using Partial Least Squares Regression (PLSR) and yields higher accuracy and precision than univariate analysis. A suite of analyzed standards of known composition are required to create a calibration model; LIBS calibrations typically require many more standards than other techniques to produce a robust multivariate calibration.
Multivariate analysis can also be used with PLSR to determine provenance or correlation without determination of any elemental abundances. This is accomplished by comparing the spectrum of the unknown sample to a set of spectra of samples of known origin, and using multivariate analysis to classify the unknown spectrum. This has been shown to be a powerful technique when the compositional variations between the possible sources of materials are not fully understood.
Multivariate analysis of LIBS spectra can also be used to compare materials to each other. For instance, it is possible to distinguish between metals that have been heat-treated and those that have not.
Literature
The following literature can be used to further explore Laser-Induced Breakdown Spectroscopy (LIBS)
LIBS Fundamentals
Cremers D.A., and Chinni R.C. (2009)
Laser-induced breakdown spectroscopy--Capabilities and limitations. Applied Spectroscopy Reviews, 44, 457-506.
Cremers D.A. and Radziemski L.J. (2006)
Handbook of Laser-Induced Breakdown Spectroscopy. John Wiley & Sons, Ltd. (Chichester), 283 pp.
Cremers D.A. and Radziemski L.J. (2006)
Handbook of Laser-Induced Breakdown Spectroscopy. John Wiley & Sons, Ltd. (Chichester), 283 pp.
Miziolek A.W., Palleschi V. and Schechter I (2006)
Laser-induced breakdown spectroscopy (LIBS): fundamentals and applications. Cambridge University Press (Cambridge), 620 pp.
Russo, R.E., Bol'shakov, A.A., Mao, X., McKay, C.P, Perry, D.L., and Sorkhabi, O., (2011)
Laser Ablation Moelcular Isotopic Spectometry. Spectrochimica Acta Part B, 66, 99-104.
Serrano, J., Moros, M. and Laserna, J.J. (2015)
Sensing signatures mediated by chemical structure of molecular solids in laser-induced plasmas. Analytical Chemistry, 87, 2794-2801.
Singh J.P. and Thakur, S.N. (2007)
Laser-Induced Breakdown Spectroscopy. Elsevier (Amsterdam), 429 pp.
Analysis of Geologic Materials
Alvey, D.C., Morton K., Harmon R.S., Gottfried J.L., Remus J.J., Collins L.M., and Wise M.A. (2010)
Laser-induced breakdown spectroscopy-based geochemical fingerprinting for the rapid analysis and discrimination of minerals: the example of garnet. Applied Optics, 49, C168-C180.
Barbini R., Colao F. Lazic V., Fantoni R., Palucci A., and Angelone M. (2002)
On board LIBS analysis of marine sediments collected during the XVI Italian campaign in Antarctica. Spectrochimica Acta Part B, 57, 1203-1218.
Bousquet B., Sirven J.-B., and Canioni L. (2007)
Towards quantitative laser-induced breakdown spectroscopy analysis of soil samples. Spectrochimica Acta Part B, 62, 1582-1589.
Clegg S.M., Sklute E., Dyar M.D., Barefield J.E., and Wiens R.C. (2009)
Multivariate analysis of remote laser-induced breakdown spectroscopy spectra using partial least squares, principal component analysis, and related techniques. Spectrochimica Acta Part B, 64, 79-88.
Cremers D.A., Ebinger M.H., Breshears C.C., Unkefer P.J., Kammerdiener S.A., Ferris M.J., Catlett K.M., and Brown J.R. (2001)
Measuring total soil carbon with laser-induced breakdown spectroscopy (LIBS). Journal of Environmental Quality, 30, 2202-2206.
Cuñat J., Fortes F.J., CabalíL.M., Carrasco F., Simó M.D., and Laserna J.J. (2008)
Man-portable laser-induced breakdown spectroscopy system for in situ characterization of karstic formations. Applied Spectroscopy, 62, 1250-1255.
De Giocomo A., Dell'Aglio M., De Pascale O., Longo S., and Capitelli M. (2007)
Laser-induced breakdown spectroscopy on meteorites. Spectrochimica Acta Part B, 62, 1606-1611.
Death D.L., Cunningham A.P., and Pollard L.J. (2008)
Multi-element analysis of iron ore pellets by laser-induced breakdown spectroscopy and principal components regression. Spectrochimica Acta Part B, 63, 763-769.
Death, D.L., Cummingham A.P., and Pollard L.J. (2009)
Multi-element and mineralogical analysis of mineral ores using laser induced breakdown spectroscopy and chemometric analysis. Spectrochimica Acta Part B, 64, 1048-1058.
Dell'Aglio M., De Giacomo A., Gaudiuso R., De Pascale O., Senesi G.S., and Longo S. (2010)
Laser induced breakdown spectroscopy applications to meteorites: chemical analysis and composition profiles. Geochimica Cosmochimica Acta, 74, 7329-7339.
Derome D., Cathelineau M., Cuney M., Fabre C., and Lhomme T. (2005)
Mixing of sodic and calcic brines and uranium deposition at McArthur River, Saskatchewan, Canada: A Raman and laser-induced breakdown spectroscopic study of fluid inclusions: Economic Geology, 100, 1529-1545.
Derome D., Cathelineau M., Fabre C., Boiron M.-C., Banks D., Lhomme T., and Cuney M. (2007)
Paleo-fluid composition determined from individual fluid inclusions by Raman and LIBS: Application to mid-Proterozoic evaporitic Na-Ca brines (Alligator Rivers Uranium Field, northern territories Australia). Chemical Geology, 237,240-254.
Dyar M.D., Tucker J.M., Humphries S., Clegg S.M., Wiens R.C., and Lane M.D. (2011)
Strategies for Mars remote laser-induced breakdown spectroscopy analysis of sulfur in geological samples. Spectrochimica Acta Part B, 66, 39-56.
Dyar M.D., Carmosina M.L., Tucker J.M., Crown E.A., Clegg S.M., Wiens R.C., Barefield J.E., Delaney J.S., Ashley G.M., and Driese S.G. (2012b)
Remote laser-induced breakdown spectroscopy analysis of East African Rift sedimentary samples under Mars conditions. Chemical Geology, 294-295, 135-151.
Ebinger M.H., Norfleet M.L., Breshears D.D., Cremers D.A., Ferris M.J., Unkefer P.J., Lamb M.S., Goddard K.L., and Meyer C.W. (2003)
Extending the applicability of laser-induced breakdown spectroscopy for total soil carbon measurement. Soil Science Society of America Journal, 64, 1616-1619.
Fabre C., Boiron M.-C., Dubessy J., Chabiron A., Charoy R., and Crespo T.M. (2002)
Advances in lithium analysis in solids by means of laser-induced breakdown spectroscopy: An exploratory study. Geochimica et Cosmochimica Acta, 66, 1401-1407.
Gondal M.A., Hussain T., Yamani Z.H., and Baig M.A. (2006)
Detection of heavy metals in Arabian crude oil residue using laser-induced breakdown spectroscopy. Talanta, 69, 1072-1078.
Gondal M.A., Hussain T., Ahmed A., and Bakry A. (2007)
Detection of contaminants in ore samples using laser-induced breakdown spectroscopy. Journal of Environmental Sciences and Health Part A, 42, 879-887.
Hark R.R., Remus J.J., East L.J., Harmon R.S., Wise M.A., Tansi B.M., Shughrue K.M., Dunsin K.S., and Liu C. (2012)
Geographical analysis of "conflict minerals" utilizing laser-induced breakdown spectroscopy. Spectrochimica Acta Part B, 74-75, 131-136.
Harmon R.S., Remus J., McMillan N.J., McManus C.E., Collins L., Gottfried J.L., De Lucia F.C., and Miziolek A.W. (2009)
LIBS analysis of geomaterials: Geochemical fingerprinting for the rapid analysis and discrimination of minerals. Applied Geochemistry, 24,1125-1141.
Horňáčková M., Grolmusová Z., Horňáčková M., Rakovský J., Hudec P., and Veis P. (2012)
Calibration analysis of zeolites by laser induced breakdown spectroscopy. Spectrochimica Acta Part B, 74-75, 119-123.
Kochelek, K.A., McMillan, N.J., McManus, C.E., and Daniel, D.l. (2015)
Provenance determination of sapphires and rubies using laser-induced breakdown spectroscopyo and multivariate analysis. American Mineralogist, 100, 1921-1931.
Martin M.Z., Wullschleger S.D., Carten C.T.Jr. and Palumbo A.V. (2003)
Laser-induced breakdown spectroscopy for the environmental determination of total carbon and nitrogen in soils. Applied Optics, 42, 2072-2077.
Martin M.Z., Labbé N., André N., Wullschleger S.D., Harris R.D., and Ebinger M.H. (2010)
Novel multivariate analysis for soil carbon measurements using laser-induced breakdown spectroscopy. Soil Science of America Journal, 74, 87-93.
McManus C.E., McMillan N.J., Harmon R.S., Whitmore R.C., De Lucia F., and Miziolek, A.W. (2008)
Use of laser induced breakdown spectroscopy in the determination of gem provenance: beryls. Applied Optics, 47, G72-G79.
McMillan N.J., Montoya C.M., and Chesner W.H. (2012)
Correlation of limestone beds using laser-induced breakdown spectroscopy and chemometric analysis. Applied Optics, 51, B1-B12.
Munson C.A., Gottfried J.L., Snyder E.G., De Lucia, F.C., Gullett B., and Miziolek A.W. (2008)
Detection of indoor biological hazards using the man-portable laser induced breakdown spectrometer. Applied Optics, 47, G48-G57.
Novontý K., Kaiser J., Galiová M., KonečnáV., Novontý J., Malina R., Liška M., Kanický V., and Otruba V. (2008)
Mapping of different structures on large area of granite sample suing laser-ablation based analytical techniques, an exploratory study. Spectrochimica Acta Part B, 63, 1139-1144.
Remus J.J., Gottfried J.L., Harmon R.S., Draucker A., Baron D., and Yohe R. (2010)
Archaeological applications of laser-induced breakdown spectroscopy: an example from the Coso Volcanic Field, California, using advanced statistical signal processing analysis. Applied Optics, 49, C120-C131.
Rosenwasser S., Asimellis G., Bromley B., Hazlett R., Martin J., Pearce T., and Zigler A. (2001)
Development of a method for automated quantitative analysis of ores using LIBS. Spectrochimica Acta Part B, 56,707-714.
Analysis of Other Materials
Bridge C.M., Powell J., Steele K.L., and Sigman M.E. (2007)
Forensic comparative glass analysis by laser-induced breakdown spectroscopy. Spectrochimica Acta Part B, 62, 1419-1425.
De Lucia F.C. Jr., and Gottfried J.L. (2011)
Rapid analysis of energetic and geo-materials using LIBS. Materials Today, 14, 274-281.
Groisman Y., and Gaft M. (2010)
Online analysis of potassium fertilizers by Laser-Induced Breakdown Spectroscopy. Spectrochimica Acta Part B, 65, 744-749.
Kraushaar, M., Noll R., and Schmitz H,-U. (2003)
Slag analysis with laser-induced breakdown spectrometry. Applied Spectroscopy, 57, 1282-1287.
Kurihara M., Ikeda K., Izawa Y., Deguchi Y., and Tarui H. (2003)
Optimal boiler control through real-time monitoring of unburned carbon in fly ash by laser-induced breakdown spectroscopy. Applied Optics, 42, 6159-6165.
Multari, R.A., Cremers, D.A., Dupre J.M., and Gustafson J.E. (2010)
The use of laser-induced breakdown spectroscopy (LIBS) for distinguishing between bacterial pathogen species and strains. Applied Spectroscopy, 64, 750-759.
Schenk E.R. and Almirall J.R. (2010)
Elemental analysis of cotton by laser-induced breakdown spectroscopy. Applied Optics, 49, C153-C160)
Wormhoudt J., Iannarilli F.J.Jr., Jones S., Annen K.D., and Freedman A. (2005)
Determination of carbon in steel by laser-induced breakdown spectroscopy using a microchip laser and miniature spectrometer. Applied Spectroscopy, 59, 1098-1102.
Related Links
For more information about Laser-Induced Breakdown Spectroscopy (LIBS) follow the links below.
http://www.arl.army.mil/www/default.cfm?page=247
http://appliedspectra.com/technology/libs.html
LIBS Applications:
http://libs.lanl.gov/
http://materialytics.com
Handheld LIBS:
https://www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/libs.html
https://www.sciaps.com/libs-handheld-laser-analyzers/
Teaching Activities and Resources
Teaching activities, labs, and resources pertaining to Laser-Induced Breakdown Spectroscopy (LIBS).


![[creative commons]](/images/creativecommons_16.png)



