For the Instructor
These student materials complement the Renewable Energy and Environmental Sustainability Instructor Materials. If you would like your students to have access to the student materials, we suggest you either point them at the Student Version which omits the framing pages with information designed for faculty (and this box). Or you can download these pages in several formats that you can include in your course website or local Learning Managment System. Learn more about using, modifying, and sharing InTeGrate teaching materials.Student Reading: Electricity, Work, and Power: The fundamentals for understanding green technology
Learning Goals: Students will be able to:
- Distinguish the differences between energy, work, and power, and provide examples of each using appropriate units.
- Provide appropriate definitions for the following electrical terms: electron, electric charge, electric potential, resistance, current, power, conductor, semiconductor, and insulator.
The student will be able to match electrical quantities/properties with the various units of measure used in electrical science (e.g. volts, amps, watts, ohms, amp-hours, kilowatt-hours, etc.) - Identify the elements of an electrical circuit.
- State the differences between parallel and series circuits, and note the effects on the electric potential (measured in volts) and current (measured in amps).
- Explain the relationship between the flow of current and magnetism, and show how this is the basis for electric motors and generators.
- Distinguish AC and DC electricity, identify the useful qualities of each, note which devices are associated with each, and describe the role of power inverters.
Energy, Work and Power
In the simplest terms, the universe consists of four things: space, time, mass, and energy. The first law of thermodynamics says energy can neither be created nor destroyed. But Einstein showed us that energy could be turned into mass and visa versa. The second law of thermodynamics says that every time energy changes forms, some of it is turned into heat. Energy comes in different forms. The most useful energy or highest quality energy is something that we can use to do work. For example, the energy of motion (kinetic energy) of water falling over a dam can be used to turn a waterwheel to grind grain or make electricity.
The lowest form of energy in terms of usefulness is heat. Yes, heat can be used to make steam and drive electric turbines. But it takes a lot of heat to do that, and that heat has to come from some other source of energy, like burning coal, or light from the sun. Physicists use the term entropy to describe the change of useful energy to less useful heat.In the simplest terms, the universe consists of four things; space, time, mass and energy. The first law of thermodynamics says energy can neither be created nor destroyed. (Although Einstein later showed that for nuclear reactions energy could be turned into mass and visa versa). Energy comes in many different forms. When energy is transferred from one object to another, or when it is transformed from one type to another, it can be used to do work. For example, the energy of motion (kinetic energy) of water falling over a dam can be used to turn a waterwheel to grind grain or make electricity.
Entropy is a measure of the distribution of energy. Concentrated forms of energy such as that stored in the nucleus of an atom, in chemical bonds, or in high-voltage electric devices are very useful for doing work. On the other hand, forms of energy that are less concentrated, such as low temperature heat, vibrations, or sound waves, are much less useful. The second law of thermodynamics dictates that whenever energy is used to do work, some of the energy is converted from a concentrated form into one that is less useful. Physicists say that as the energy spreads out or disperses, the entropy is increasing. One result of the second law of thermodynamics is that no process can ever convert 100% of the energy into useful work.
What is energy? It is useful to categorize energy into two lists. Kinetic energy is the energy of something in motion. Falling water (responding to the force of gravity), light from the sun, electrons flowing in a wire (electricity), a bicycle in motion, using your muscles to move your eyes while reading this are all examples of kinetic energy. Potential energy is that which is stored and ready to be changed to kinetic energy. This includes the water held back by a dam, the electrical charge stored in a battery, the chemical energy stored in fats and sugars, and the chemical energy stored in gasoline and coal.
In the diagram of a hydroelectric plant, the water flowing down the penstock has kinetic energy. That kinetic energy is being used to turn the turbine connected to the electric generator. The water stored behind the dam has potential energy or stored energy. Note that it is the force of gravity pulling on the water that provides the energy in each case.
Force
When energy is applied to an object we think of it as a force. Some forces require contact between two objects, and others work at a distance. Forces that require contact include pushing, pulling (tension), and friction. Forces that work without direct contact between objects include gravity, magnetism, and electrical force. The standard unit of force is named for Sir Isaac Newton, a father of physics. One Newton (1 N) = the amount of force to accelerate 1 kg of mass one meter per second2. Or, 1 N = (1 kg x 1 m)/s2.
Work
We use energy to do work. The simplest way to think about work is in terms of moving an object. When a force (mass times acceleration) is applied to an object and causes that object to be moved, the distance moved is the work that is done. But we use energy to do more kinds of work than moving furniture or automobiles. Work is also done when we use sunlight or natural gas to heat our houses, when we use electricity to light our rooms, or when we use a peanut butter and jelly sandwich to power our brain cells.
Since energy comes in different forms, it is not surprising that there are different ways to measure it. It is hard keeping track of all the different units of energy. Look at the table below to see some of the units and the relationship to the joule, which is the gold standard of energy measurements. It is named for James Joule, a 19th century brewer who showed the equivalence of mechanical work and heat. One joule is about equal to the amount of energy needed to lift a 100 g apple 1 meter (3.3 feet).
The apparatus pictured was used by James Joule to demonstrate equivalence of mechanical work and heat. He calculated the work done by the pull of gravity on the weight. That pull turned the paddle wheels, which mixed the water in the insulated container. The water was warmed by the mixing, showing that heat = work.
Power
Power is a measure of how much energy is used over a period of time. For that we can use the watt. James Watt was a pioneer in understanding the physics of energy and developed one of the earliest successful steam engines. He loaned us his last name for this unit.
Shown is an image of the steam engine co-developed by James Watt for pumping water out of flooded coal mines in England.
A watt is one joule of energy expended in one second. So a watt incorporates both the energy expended, and the time that it was expended over. By analogy, you can get one gallon of water from a dripping faucet in one hour, or from an open faucet in 15 seconds. In the end you still get a gallon of water, but the flow of the water into the bucket is much faster in the second case. So the aspect of time is important. We use the term power to incorporate both the amount of energy and how fast it was delivered. The joule is the energy term, and the watt is the power term.
How big is a watt of power? Tossing a 100 g apple up in the air 1 m (3.3 feet) takes 1 watt of power. The notebook computer you may be using to read this is using about 5â€"50 watts, depending if you have music running in the background, or other applications cranking away. An old fashioned incandescent light bulb rated at 100 watts will use one 1 kilowatt hour of electricity if left on for 10 hours. A kilowatt is 1,000 watts, and abbreviated as a kW. 10 hours x 100 Watts = 1 kWh. Note the difference between a kW and kWh. A kW is a measure of power, while the kWh is a measure of how much energy was used in total.
Are you confused about kW and kWh? This is the trick. Remember that the watt is a joule/sec. So a watt or a kilowatt already has time built into it. It is energy/time. It is power, the rate of how fast energy is used. But power does not tell you how much energy was used over a period of time. To get that you have to multiply the power times the time. Then the time units should cross out. Alas, the convention is to leave the hour in place â€" silly but that is the way it is done. 1 kWh = 1kW x 1 h.
Here is an example. My house has a photovoltaic system (solar electric power) that under ideal conditions of a nice sunny cool day is rated to produce at the rate of 4 kW. In 4 hours it would make:
4 kW x 4 hours = 16 kWh worth of electricity. On a partly cloudy day the system might be operating at half power or 2 kW of output. At that rate I would need 8 hours to make the same 16kWh I made on the sunny day; 2kW x 8 hours = 16 kWh.
At rest, a typical human is using energy at the rate of 80 W to power the body functions of life (called resting metabolism). An adult man might eat about 2,000 killocalories a day. One kcal = 1.163 Wh. So that 2,000 kcal diet will provide 2,326 Wh or 2.326 kWh. If the person just rested in bed for 24 hours, he would burn 80 W x 24 h = 1920 Wh or 1,920 kWh. If that guy stays in bed and continues to eat like that he will end up consuming 2.326 kWh â€" 1.920 kWh = 0.406 kWh more then he uses, and that will be stored as fat. A pound of fat is equal to about 3,500 kcal (4.070.5 kWh). So in ten days he might add an extra pound. A vigorous bike ride uses energy at the rate of 200 W. So he should consider a nice two-hour bike ride to stay trim (0.2 kW for biking x 2 hours = 4.0 kWh).
Summary of Force, Work and Power
Force = Energy applied to an object(Measured in Newtons).
Work = Force X Distance, or the amount of heat transferred (Measured in Joules or calories).
Power = Work/Time (Measured in Watts)
Various Energy Units
1 calorie (thermochemical) = 4.184 J
1 Btu = 251.9958 calories
1 Btu (thermochemical) = 1054.35 J
1 kilowatt-hour (kWh) = 3.6 x 106 J
1 kilowatt-hour (kWh) = 3412 Btu (IT)
1 therm = 100,000 Btu
1 electron-volt = 1.6022 x 10-19 J
Electricity and Magnetism
Now that you have a good idea about energy, work, and power it is time to get charged-up to study electricity! The ancients had a vague notion of electricity through their life experiences. Fishers who caught various kinds of "electric fish" were in for a shock when handling them. Others felt the effects of static electricity from their woolen garments. The Egyptians saw the connection between electric fish and lightning. But it was not until about 1600 that serious scientific study of electricity began. Through the efforts of many different researchers, a good understanding of electricity and how to use it was developed by the end of the 19th century.
Recall that all matter is comprised of atoms. And atoms consist of several major particles: electrons that have a negative charge, protons that have a positive charge, and neutrons that have no charge. Electricity can be thought of as the flow of electrons through a conductor like a copper wire. In reality, it is not a flow of electrons, but an impulse that is passed along the wire.
Good conductors, like metals, easily permit the flow of electricity. They have electrons in their outer orbitals that are easily engaged. Poor conductors are called insulators, and they do not allow the easy flow of electricity. Even the best conductors offer some resistance to the flow of electricity. Such resistance is measured in units called Ohms. Glass is a good insulator and therefore a poor conductor.
A third class of compounds is semiconductors. They respond to changing conditions to turn on or off the flow of electricity. Semiconductors often contain a mixture of silicon and metals. Wafers of these semiconductors are at the heart of the "chips" in a computer, and are also the basis for LED lights and photovoltaic (solar) cells.
Panels of photovoltaic cells used to make electricity from sunlight are made of semiconductors.For electricity to flow there must be a closed circuit. Electrons have to start out at a high-energy state and end up at a low-energy state. Below is a diagram of a simple circuit. Note that electricity flows from the high-energy end of the battery through the lamp and then back to the low-energy end of the battery. When the switch is opened the flow of electricity stops.
It is simple to think of electricity as an electron (or electron-sized impulse) flowing through a conductor. But in practice a single electron is way too small and carries way too little energy to do any real work. However, groups of electrons flowing together can pack a big jolt! A coulomb is 6.24 x 1018 electrons. And an ampis a flow of one coulomb per second through a conductor. So amps measure the rate of electricity flow. We call the flow of electricity current.
Electricity does not all flow with the same force. To understand this, think about the pressure or force of water coming out of a pipe. If the pipe is attached to a reservoir at the top of a tall building, the water will have a lot more pressure than if the tank is just a foot (30 cm) or so above the pipe. It works the same way for electricity. The "pressure" of the electricity is electric potential. Electric potential is the amount of energy available to push each unit of charge through an electric circuit. The unit of electric potential is the volt. A volt is equal to a joule per coulomb. Thus, if a car battery has an electric potential of 12 volts, then it can provide 12 joules of energy for each coulomb of charge that it delivers to the starter motor. Likewise if an outlet in your home has an electric potential of 120 volts, then it can provide 120 joules of energy for each coulomb of charge that is delivered to a device plugged in at the wall. (Note: the quantity "electric potential" is sometimes called by several different names, including voltage, potential difference, and electromotive force. For clarity purposes, we will always refer to the electric potential, which is measured in the units of volts). High-voltage electrons arrive back to the "ground state" with more energy than low-voltage electrons.
A volt is the force needed to move one amp through a conductor that has 1 ohm of resistance.
You are thinking, "There seems to be a relationship between amps, volts, and ohms" â€" and you are right! Electric potential = Current x Resistance. This is Ohm's law and is usually written as: E = I x R. E is electric potential measured in volts, I is current measured in amps, and R is resistance measured in ohms.
The electrons flowing through the resistance of a wire are doing work. Two kinds of work performed by the current are really useful. If there is a lot of resistance in the wire, it will cause much of the work to be in the form of heat. Think electric toaster, hair dryer, or space heater.
The second really important kind of work done by the current flowing through a wire is the creation of a magnetic field. Hopefully you have played with permanent magnets when you were a kid. You know magnets have two poles, one called north and the other called south. This naming comes from the use of magnets in compasses for finding direction. You know that the like ends of magnets repel each other, while the opposite ends attract each other. Now, when electrical current flows through a wire, the wire becomes like a magnet in that it has a magnetic field. However, unlike permanent magnets, the magnetic field can be turned off by stopping the flow of current. This property is the basis for how electric motors function. The current going through the windings of wires in an electric motor causes the magnetism to be turned on. This then causes the motors to spin, being pulled and pushed by the attraction and repulsion of electromagnets.
The work done by the current over time is called power. Power is measured in watts. But you know that already! Recall that above you learned that a typical human at rest is burning 80 watts.
For electricity;
1 Watt = 1 Amp x 1 Volt.
The equation can be rearranged to calculate the current being produced;
1 Amp = 1 Watt/1 Volt.
To summarize.
Amps measure the amount of electricity flowing over time (current).
Ohms measure the resistance to flow.
Volts measure the amount of energy available to push each unit charge.
Watt is the measure of power, or work that gets done over time.
You know that Ohm's law states the relationship between E, I and R. But how much work is being done? That is expressed as Power. Power = Electric potential x Current, or P = E x I. This formula makes the point that the power depends on both the amount of electricity being delivered and how much force there is behind it. For example, a small solar panel might produce 18 volts and 2 amps. Its power production would be 18 volts x 2 amps = 36 watts. Now another solar panel could be constructed to produce 9 volts and 4 amps. Its power production would be 9 volts x 4 amps = 36 watts. Just the same as the other one!
Circuits
Equipment that produces and uses electricity is connected in a circuit. The equipment can be arranged either in series or in parallel. Look at the circuits below to see the consequences of using series versus parallel arrangements. For photovoltaic (PV) cells, each cell can produce only about 0.6 volts. Since most applications require higher voltage, PV cells must be placed in series to produce the desired results.
Electric Motors and Generators
Recall that part of the work done by electricity occurs when it passes through a wire to create a magnetic field. Hans Christian Oersted discovered that in 1820. A year later Michael Faraday showed that the magnetic field around the wire can be used to create electromagnets that can be cleverly arranged to make an electric motor.
Note the image of the electromagnet made by wrapping insulated wire around an iron nail. The iron nail concentrates the magnetic field created by the current in the insulated wire. The insulation prevents the iron nail from shorting the circuit.
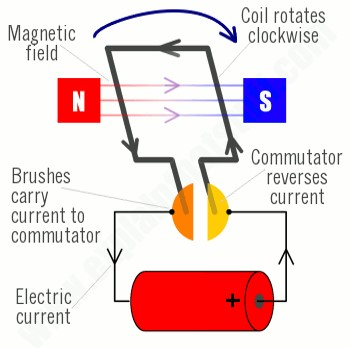
The diagrams below show how an electric motor works. Note that at each half rotation the contacts in the commutator reverse the current to keep the motor spinning in the same direction.
Michael Faraday did not perfect the electric motor, but he did discover an important property of electromagnetism that led to another great invention, the electric generator. Faraday discovered in 1831 the principle of magnetic induction. He found that by passing a magnet along a wire it caused the flow of electricity in a closed circuit. This led to the development of electric generators. The first successful commercial designs appeared around 1860. An electric generator is essentially an electric motor that is spun by some outside force, and in response produces induced current. Hybrid electric cars like the Toyota Prius do exactly that. The electric motor is powered by a battery when the accelerator pedal is pressed. When the pedal is released, the momentum of the car acts through the rotating wheels to turn the motor, causing the motor to be a generator, creating electricity to recharge the battery.
Alternating Versus Direct Current Electricity
So far we have only considered one kind of electricity, Direct Current (DC). This is what is produced by batteries, solar panels, and DC generators. For DC electricity the current always flows in the same direction. The other kind of electricity is Alternating Current (AC). As the name indicates, the current switches direction in the wire on a regular cycle. AC electricity is what comes to our homes through the power grid. It is produced by AC generators. An AC generator is wired differently from a DC generator. Remember that in a DC generator or motor there is a commutator or rectifier that switches direction of current in coils of the armature (the part that rotates). An AC generator uses slip rings instead of the reversing commutator. As such, with each half revolution of the generator, the induced current changes directions.
The output for an AC generator produces a sine wave as the electricity surges back and forth in the circuit. The reversal of current is fast. In the United States the standard for the power grid is 60 Hertz (switching back and forth 60 times a second).
The diagram to the right shows a sine wave produced by an AC generator. Above 0 volts the electricity is flowing one direction, and below 0 volts it is going the other. The Y-axis is voltage and the X-axis is time.
Short video on the difference between DC and AC generators and motors
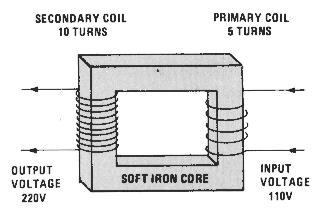
An advantage of using AC power is that it is easy to step up or reduce the voltage in different parts of the delivery system grid. This is done by transformers. A transformer consists of two side-bye-side coils, one large and one small. Both coils share a common iron core. AC current going through a small primary coil will by magnetic induction create a current of higher voltage in the larger secondary coil. And the reverse is also true, if the primary coil is the larger one, the smaller secondary coil will have a lower voltage output.
Why bother increasing and decreasing voltage? Remember V=I x R. Transmission of electricity over great distances results in loss of energy to heat due to the resistance of the wires. To prevent this, the voltage is increased, requiring less current and less loss to heat. The voltage is dropped back down when reaching your house. High-tension power lines may carry electricity at 765 kV (that is 765,000 volts!). What get from a wall socket is 120 volts.
Going between AC and DC Electricity
Since we use both AC and DC electricity, it is important to be able convert one into the other. This job is done by a device called a power inverter. Many household appliances run on the AC delivered by the power grid. Refrigerators, air conditioners, incandescent and florescent lights, vacuum cleaners, hair dryers and washing machines all use AC directly. Electronics such as computers, television sets, and cell phones require DC. The devices generally have the inverter built into the AC power cord. The wire leading away from the inverter carries the DC required by the device.
Power inverters are also useful to turn DC into AC. Such devices allow one to use the 12 volt DC of a car to power a laptop computer. Houses that use photovoltaic panels to harness solar energy to make electricity must also convert their production to match the AC, if the systems are tied to the power grid.
Both types of inverters use electronic circuits to make the transition in electricity. The theory of their operation is beyond the scope of this basic unit. But you should know that power inverters obey the second law of thermodynamics. So energy is lost to heat during the conversion process. But modern inverters may achieve efficiencies as high as 95%.
A power inverter that converts the DC from solar panels to AC for a grid-tied PV system is shown.
Storing and Producing Electricity with Batteries
Batteries convert the potential energy of chemicals into the kinetic energy of electricity. Benjamin Franklin coined the term "battery" to describe the stacks of metal-coated glass plates he used to store energy. But what he had we would call capacitors today. Batteries work by pairing two chemical materials together that have different affinities for electrons. Anode materials prefer to lose electrons, while cathode materials prefer to gain them. The electrodes of a battery are immersed in a solution containing positive and negatively charged ions called an electrolyte. When connected in a circuit, electrons flow from the anode to the cathode. At the same time, negatively charged ions in the electrolyte move from the cathode to the anode in order to maintain charge neutrality, and thereby complete the electric circuit.
In a rechargeable battery, the reactions at the anode and cathode can be reversed by using electrical energy to supply a current that pushes electrons in the opposite direction - from the cathode to the anode. This restores the original condition of the two electrodes. Your laptop computer, cell phone, and automobile battery are all examples of rechargeable batteries. Modern batteries use combinations of various types of metal and metal oxide compounds formed from elements that include carbon, cadmium, cobalt, lithium, manganese, nickel, lead, and zinc for improved performance.
A simple battery using and acid fruit and two different metals (bronze and steel alloys).
Exercises Exercises for Module 1 (Microsoft Word 2007 (.docx) 17kB Jul12 17)
1. Create a circuit using two batteries in series and a light bulb. Use a Digital Multi Meter (DMM) to measure the electrical potential in volts between the positive and negative terminals in the circuit. Now add a second light bulb to the circuit in series with the first. How does the brightness of each bulb compare to when there was only one in the circuit? Use a volt meter to measure the voltage between the positive terminal of the battery and the wire just after the first bulb, and then just after the second bulb. Record the results. Now create a circuit with the two light bulbs in parallel. Record the brightness and the voltage across each bulb.
Explain your results.
Simple circuit with one bulb
| Bulb brightness | |
| Voltage drop (V) |
Circuit with two bulbs in series
| First bulb | Second bulb | |
| Bulb brightness | ||
| Voltage drop (V) |
Circuit with two bulbs in parallel
| First bulb | Second bulb | |
| Bulb brightness | ||
| Voltage drop (V) |
2. Build five elector magnets, each with different lengths of wire wrapped around the iron nails: 10 cm, 20 cm, 30 cm, 40 cm, and 50 cm. In each case there should be an extra 10 cm on each end of the wire so you can connect it to a battery. So the "10 cm" coil will actually be made using a 30 cm length of wire, and so forth. Connect each magnet to a battery and add as many paper clips as possible to a magnetic chain from the tip of the nail. Record the maximum number of paper clips in each case. Then make a graph of the maximum number of paper clips held versus the length of wire used to make the windings. Explain why the graph looks like it does.
| Length of wire in coil (cm) | 10 | 20 | 30 | 40 | 50 |
| Max. no. of paper clips |
3. Build a simple motor from the kit provided. Be sure to pay attention to the instructions on how to remove the insulation on opposite sides of the wire that contacts the battery clips. Once you get your motor to rotate, perform the following experiments.
a. Note the direction the motor turns. Can you get it to go in the opposite direction? Explain.
b. Now remove the magnet and flip over. Then restart your motor. Does it turn in the same direction as before? Why?
c. Now turn the battery around and restart the motor. Did the direction of rotation remain the same? Explain why.
d. Think of the electric motor as a system. Identify the source of energy and the fate of that energy in the spinning motor system. In your answer use the following terms: electrochemical energy, kinetic energy (energy of movement), and heat. Draw the circuit you created to run the electric motor. Put on your systems thinking hat.
- Identify each component of the system.
- Trace the flow of energy through the system. Be sure to show where it goes from electrical current to magnetic energy, kinetic energy, and heat.
- Take a picture of your diagram and include it in your report.
Is the electric motor a closed system (all the energy stays in the system) or is it an open system (some energy exchanges with the environment)?
4. Use a piece of citrus fruit to create a battery. Put a copper penny in one side of the fruit and a steel paper clip on the other side. Measure the voltage with a DMM. Record the result: ______.
Now try to use the fruit battery to light an LED bulb. Does it work? Explain what is creating the electricity.
References
Electromagnets and Faraday's law
- Magnetism: Electromagnets YouTube video from CHMnanoed
- Physics - Electromagnetism: Faraday's Law YouTube video from EducationCommonsRW's channel
Electric motor and generator
- Magnetism: Motors and Generators YouTube video from CHMnanoed
AC induction motor
- Induction Motor How it works Vimeo video from agmlabs.com
Transformers
- Magnetism: Transformers YouTube video from CHMnanoed
AC/DC Inverters
- Introduction to Grid Tie Inverters â€" Part 1 YouTube video from Gary Straughan
- Introduction to Grid Tie Inverters â€" Part 2 YouTube video from Gary Straughan
- Understanding Power Supplies, Generators, AC, and DC YouTube video from Grants Pass TV Repair
How Batteries Work
- How a Lead Acid Battery Works YouTube video from Engineerguy
![[reuse info]](/images/information_16.png)




![[creative commons]](/images/creativecommons_16.png)