Advanced Modeling Programs: TWQ
What is it?
TWQ is an easy to use, Windows-based program that allows you to calculate the position of phase equilibria in P-T, T-XCO2, and P-XCO2 space. You can also use it to calculate various activity diagrams. It is maintained by Rob Berman at the Geological Survey of Canada (GSC) and is available for free from the GSC website:
Principles
The underlying philosophy behind using TWQ over "classical" geothermobarometry calibrations published in the literature, is that it uses an internally consistent thermodynamic database (Berman, 1991) to calculate P's and T's. For example, if you combine the Ferry and Spear (1978) calibration of the garnet-biotite thermometer with the Ghent (1976) barometer, it is very much like comparing apples and oranges because the endmember (fundamental) thermodynamic data derived from these calibrations are different.
Applications
TWQ allows you to calculate the positions of reactions in P-T-X(composition) space involving pure and solid-solution phases including H2O and CO2.
Strengths and limitations
Ease of use is the strength of winTWQ. Following the tutorial below, new users can be up and calculating in a relatively short period of time. The primary drawback to winTWQ is that it only runs in Microsoft Windows. However, I run Windows on my Intel-based Mac using parallels and it works great. Another potential drawback is the number of phases in the Berman (2007) database is limited compared to Holland and Powell (1998); however, most of the common phases used in geothermobarometry are available.
Worked Examples
The purpose of this tutorial is to walk new users through the basic use of winTWQ. By the end, you will be able to 1) calculate and plot the Al2SiO5 phase diagram, and 2) use mineral chemistry from Mt. Moosilauke, New Hampshire (Hodges and Spear, 1982) to calculate the positions of all the possible reactions for this sample in the KCFMASH system including the familiar garnet-biotite exchange reaction (Ferry and Spear, 1978) and GASP reaction (Ghent, 1976).
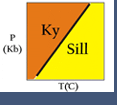
Creating the Al2SiO5 Phase Diagram
- Open winTWQ. A dialog box appears asking if you want a batch run, click continue.
- Another dialog box appears giving you choices of databases. Use the default by clicking continue.
- Another dialog box appears. For now, use all the defaults and click Next.
- Now you can select the type of diagram you want to plot. Let's make a P-T plot. Select P-T and click Next.
- The next dialog box asks for the chemical system you want to use. Si and O are already entered for you. Let's add aluminum. Click in the Elements box, type a space and then "al". Note that elements don't need to be capitalized and there must be spaces between elements. Now set the T and P range you want to plot. Let's leave the P defaults but change the T range to 200-900°C. Be sure to include the decimal point after 200. and 900. Finally, select "Stable Curves" under Plot Output. Click Next.
- Based on the elements you chose, a list of minerals appears in the next dialog box. Choose Andalusite, Kyanite, and Sillimanite by clicking on each mineral name. Click Next.
- A list of possible reactions is given. Select "Calculate all reactions" in the lower left, and then click Next.
- A summary dialog box appears. Click Exit, and the program quits.
- To view your result, run wplot.exe; from the file menu, open the file labeled Plot.plt. It should look like Figure 1.
Using Mineral Compositions and Geothermobarometry
Here we use TWQ to calculate the position of the garnet-biotite thermometer (Ferry and Spear, 1978) and the GASP barometer (Ghent, 1976). We will use mineral compositions reported by Hodges and Spear (1982) from Mt. Moosilauke, New Hampshire. The mineral assemblage of the sample is garnet + biotite + sillimanite + muscovite + plagioclase + quartz.
- Copy the file mtm90a.oxi ( 417bytes Mar31 07) to your winTWQ folder. Open the file using a text editor (e.g. WordPad). It should look like this:
- The first line can be anything you want. The second line gives the format of the following lines. After "Sample", the oxide list can be in any order you wish. Just make sure that the mineral compositions that follow use the same order. Note: if you organize your probe data in a spreadsheet, be sure to save your text file as comma delimited, and be sure to replace the .csv extension with .oxi. Also note that the first letter of the "Sample" designator tells the program the type of mineral the oxide list refers to. To see a complete list of the minerals you can put into a .oxi file, open the cmptest.oxi file. The first line of this file gives the minerals and their one-letter designators.
- Start the program winCMP.exe. This program uses the .oxi file to calculate mineral formulae used by winTWQ.
- Enter the name of your .oxi file, in this example, mtm90a. Go ahead and review the defaults, and leave as is. Then click the button labeled "version 2.32". This creates a file labeled mtm90a.cm3. Now you are ready to run winTWQ with the mineral compositions from Mt. Moosilauke.
- Start winTWQ, and click through the first four dialog boxes using the default settings. When you get to the box asking for the elements, enter O, Si, Al, Fe, Mg, K, Ca, H. These are the elements that make up the KCFMASH system needed to describe the garnet-biotite and GASP reactions.
- Set the P-T range to what we used above (P: 500-10,000 bars; T: 200-900°C), and select Stable Curves. Click Next.
- Select the minerals you need to describe the garnet-biotite and GASP reactions: almandine, annite, anorthite, grossular, phlogopite, pyrope, a-quartz (alpha quartz), and sillimanite. Let's include kyanite and andalusite so we have the Al2SiO5 phase diagram for reference. Click Next.
- Make sure Yes is selected for the solution models, then enter the name of your CMP file near the bottom of the dialog box: mtm90a.cm3. Click Next.
- A list of all the possible reactions that can be generated based on your inputs is shown. Select Calculate all reactions and click Next. One more box appears telling you its done, click Exit.
- View your plot.plt file using wplot (Fig. 2). Note that the intersection between the garnet-biotite thermometer and GASP barometer occurs in the kyanite field, yet this rock contains sillimanite! However, also note that the intersection is very close to the kyanite-sillimanite curve, so perhaps we are doing pretty well.
Using the Restart File and Calculating All Equilibria
Berman (1991) suggests that calculating all possible reactions, or some subset of reactions (Berman, 2007), can be used as a possible test for equilibrium. That is, if 1) all the minerals in a rock were at one time in equilibrium, and mineral compositions have not changed since that time, 2) the mineral compositions are perfectly known/measured, and 3) the thermodynamic data for all the minerals are correct, then all the possible reactions calculated of a given mineral assemblage should intersect at a single point. Of course, for real rocks and mineral databases, one or more of these assumptions is violated. Having said that, it is a useful exercise to go through, and easy to do in winTWQ.
The following assumes you completed the tutorial above "Using Mineral Compositions and Geothermobarometry". This allows us to use the Restart file. Using the Restart file is a quick method to rerun winTWQ if you don't plan to change the chemical system.
- Open the restart.res file in a text editor. It should look something like this:
- In the fourth line, change Y to N. This tells winTWQ to plot the metastable extensions of the calculated reactions.
- Note the 0's and 1's in the first column of the mineral list. A "0" (zero) tells winTWQ to include the phase, and a "1" to exclude. Scroll down the mineral list, and find muscovite; change the "1" to "0". We include muscovite because it is part of the mineral assemblage of this rock. Also, remove andalusite and kyanite by changing the "0" to "1". We remove these phases because they are not part of the mineral assemblage. Save and close the Restart.res file.
- Start winTWQ; when you get to the third screen (the screen that lists the data files), click "Yes" under "Use Restart file?". Click Next. TWQ will run and generate an updated version of the Plot.plt file. Note that before you click next, you can type in a new name for the plot file if you don't want to overwrite the existing file.
- Run wplot.exe and take a look at your new plot file. It should look like Figure 3.
- TWQ calculated six possible reactions for this sample based on the KCFMASH system and the mineral assemblage we chose:
- Reactions 1 & 3 are the familiar GASP and garnet-biotite reactions, and the other four reactions contain muscovite. However, of these six reactions, only three are independent (Berman, 1991). That is, the other four reactions are linear combinations of the three independent reactions. Regardless, note that all the reactions in Figure 3 intersect in a relatively small region of PT-space, suggesting that the phases in this rock record equilibrium compositions.
References
Berman, R.G. (1988) Internally-consistent thermodynamic data for minerals in the system Na2O-K2O-CaO-MgO-FeO-Fe2O3-Al2O3-SiO2-TiO2-H2O-CO2. Journal of Petrology, 29, 445-522.
Berman, R.G. (1991) Thermobarometry using multi-equilibrium calculations: a new technique, with petrological applications; in, Quantitative methods in petrology: an issue in honor of Hugh J. Greenwood; Eds. Gordon, T M; Martin, R F. Canadian Mineralogist v. 29, 833-855.
Berman, R.G. (2007) winTWQ (version 2.3): a software package for performing internally-consistent thermobarometric calculations. Geological Survey of Canada, Open File 5462, (ed. 2.32) 2007, 41 pages.
Ferry, J.M. and Spear, F.S. (1978) Experimental calibration of the partitioning of Fe and Mg between biotite and garnet. Contributions to Mineralogy and Petrology, 66, 113-117.
Ghent, E. D. (1976) Plagiociase-garnet-Al2SiO5-quartz: a potential geobarometer-geothermometer. American Mineralogist, 61, 710-714.
Hodges, K. V. and Spear, F. S. (1982). Geothermometry, geobarometry and the Al2SiO5 triple point at Mt. Moosilauke, New Hampshire. American Mineralogist, 67, 1118-1134.
Holland, TJB, & Powell, R, 1998. An internally-consistent thermodynamic dataset for phases of petrological interest. Journal of Metamorphic Geology 16, 309-344.
Related Links
TWQ website -- for downloading TWQ
Dmitry Dolivo-Dobrovolsky Webpage
- TWQ_View: the program for viewing and work with diagrams generated by the TWQ program of R.G. Berman (1991) used for multi-equilibrium geothermobarometry. TWQ_View is a handy ©MS Windows freeware for viewing and basic work with diagrams generated by the TWQ(link is external)/winTWQ(link is external) program package of R.G. Berman, T.H. Brown, E.H. Perkins and J. Ellwood.
- TWQ_Comb is a ©MS Windows freeware for automatic generation of all possible combinations of selected microprobe analyses and for their processing by the [win]CMP.EXE and [win]TWQ.EXE programs (from the TWQ(link is external) or winTWQ(link is external) packages of R.G. Berman et al.) running in the batch mode.
Teaching Activities
- Problem Set: TWQ Exercise - Dexter Perkins, University of North Dakota; Students use TWQ to calculate a phase diagram that includes all reactions that can take place involving 9 minerals in the MgO-Al2O3-SiO2 system.
- Multi-equilibrium Thermobarometry Lab (Microsoft Word 53kB Mar29 07) - This Excel-based one week exercise, provided by Dave Pattison at the University of Calgary, includes problems sets involving multi-equilibrium thermobarometry using TWQ and ThermoCalc's 'AvePT' module ('Optimal thermobarometry').